Advancements and Evaluation of SERS Substrates for Sensitive Detection of Environmental Pollutants
Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique for the trace-level detection of environmental pollutants, offering unparalleled sensitivity, molecular fingerprinting capability, and potential for on-site analysis.
Advancements and Evaluation of SERS Substrates for Sensitive Detection of Environmental Pollutants
Abstract
Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique for the trace-level detection of environmental pollutants, offering unparalleled sensitivity, molecular fingerprinting capability, and potential for on-site analysis. This article provides a comprehensive evaluation of SERS substrates, from fundamental enhancement mechanisms and innovative nanomaterial designs to their practical application in detecting pesticides, heavy metals, biotoxins, and organic contaminants. Tailored for researchers, scientists, and drug development professionals, the review systematically compares substrate performance, addresses key challenges in reproducibility and quantitative analysis, and explores the integration of computational design and artificial intelligence. By synthesizing foundational knowledge with cutting-edge methodological advances and validation frameworks, this work serves as a critical resource for navigating the development and application of SERS technologies in environmental monitoring and public health protection.
Principles and Design of SERS Substrates: From Plasmonics to Nanomaterial Innovation
Electromagnetic and Chemical Enhancement Mechanisms in SERS
Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique that transcends the sensitivity limitations of conventional Raman spectroscopy. By leveraging nanostructured materials, primarily noble metals, SERS can amplify inherently weak Raman signals by factors ranging from 10ⴠto as high as 10¹¹, enabling single-molecule detection in ideal configurations [1] [2]. This extraordinary enhancement capability makes SERS particularly valuable for detecting trace environmental pollutants, where sensitivity and specificity are paramount. The signal amplification in SERS originates from two distinct but potentially synergistic mechanisms: the electromagnetic enhancement mechanism (EM) and the chemical enhancement mechanism (CM) [2]. Understanding the interplay between these mechanisms is crucial for researchers evaluating SERS substrates for environmental applications, as it directly influences substrate selection, experimental design, and analytical performance.
The evaluation of SERS substrates for environmental pollutant detection presents unique challenges, including complex sample matrices, low analyte concentrations, and the need for reliable field deployment. This comparison guide objectively examines the fundamental enhancement mechanisms, their relative contributions to SERS performance, and practical implications for environmental research. By synthesizing current research trends and experimental data, this guide provides a framework for selecting and optimizing SERS substrates based on their enhancement characteristics and application requirements.
Fundamental Principles of SERS Enhancement
Electromagnetic Enhancement Mechanism
The electromagnetic enhancement mechanism (EM) is widely recognized as the dominant contributor to SERS intensity, typically accounting for 10â´ to 10â¸-fold signal amplification [1] [2]. This mechanism originates from the excitation of localized surface plasmon resonance (LSPR) in plasmonic nanostructures, primarily composed of noble metals such as gold (Au) and silver (Ag) [2]. When incident light interacts with these metallic nanostructures at frequencies matching their collective electron oscillation frequency, it induces resonant oscillations known as surface plasmons. This resonance creates dramatically enhanced electromagnetic fields at specific locations on the nanostructure surface, particularly at sharp tips or within narrow gaps between particles—regions famously termed "hot spots" [1] [3].
The electromagnetic enhancement process involves two complementary effects: first, the enhanced local field amplifies the excitation of Raman scattering when light interacts with molecules located within these hot spots; second, the same enhancement mechanism amplifies the Raman-shifted emission from the molecules [2]. Since both the incoming and outgoing processes are enhanced, the overall Raman intensity scales approximately with the fourth power of the local field enhancement (E/Eâ‚€), explaining the extraordinary amplification factors achievable through EM [3]. The EM mechanism is largely non-specific, depending primarily on the molecular proximity to enhanced fields rather than specific chemical interactions, making it broadly applicable across various analyte types.
Table 1: Key Characteristics of Electromagnetic Enhancement
| Characteristic | Description | Implication for SERS Performance |
|---|---|---|
| Enhancement Factor | 10ⴠ- 10⸠| Provides major contribution to overall SERS signal |
| Distance Dependence | Sharp decay (~dâ»Â¹Â²) | Requires analyte proximity to substrate surface |
| Material Dependence | Noble metals (Ag, Au, Cu) | Limited to plasmonic materials |
| Specificity | Non-specific | Broadly applicable to various analytes |
| Spatial Distribution | Localized at "hot spots" | Signal heterogeneity requires careful sampling |
Chemical Enhancement Mechanism
The chemical enhancement mechanism (CM) provides a secondary but significant contribution to SERS signals, typically offering 10¹ to 10³-fold enhancement [2]. Unlike the physically-based EM mechanism, CM involves direct chemical interactions between the analyte molecules and the substrate surface at the quantum mechanical level. This mechanism primarily arises from charge transfer between the energy levels of the metal substrate and the adsorbed molecules, which creates new electronic states and resonances that increase the Raman scattering cross-section [2] [3].
The chemical enhancement process requires direct chemical adsorption of target molecules onto the substrate surface, often through specific functional groups that facilitate charge transfer. The enhancement depends critically on the molecular orbitals of both the adsorbate and substrate, making it highly specific to particular molecule-substrate combinations [3]. While CM provides substantially lower overall enhancement compared to EM, its importance lies in its ability to selectively enhance specific analytes based on their chemical properties, potentially improving detection specificity in complex environmental samples. Additionally, CM exhibits a much weaker distance dependence than EM, maintaining effectiveness for directly adsorbed molecules even outside the strongest electromagnetic hot spots.
Table 2: Key Characteristics of Chemical Enhancement
| Characteristic | Description | Implication for SERS Performance |
|---|---|---|
| Enhancement Factor | 10¹ - 10³ | Secondary contribution to SERS signal |
| Distance Dependence | Weak decay | Effective for directly adsorbed molecules |
| Material Dependence | Various semiconductors/metals | Broader material options including MXenes |
| Specificity | Highly specific | Selective enhancement based on chemistry |
| Adsorption Requirement | Direct chemical bonding | Requires specific molecular functionalities |
Synergistic Effects and Combined Enhancement
In practical SERS applications, electromagnetic and chemical enhancement mechanisms do not operate independently but rather exhibit complex synergistic interactions [2]. The combined enhancement factor is not merely the product of individual EM and CM factors, as both mechanisms can influence each other through various interfacial interactions. For instance, chemical bonding can alter the local electronic environment of plasmonic nanostructures, potentially modifying their plasmonic properties and thus the electromagnetic enhancement. Conversely, strong electromagnetic fields can influence charge transfer processes, affecting the chemical enhancement component.
This synergy is particularly evident in hybrid SERS substrates that combine plasmonic metals with functional materials such as semiconductors, graphene, or metal-organic frameworks (MOFs) [2]. In these systems, the plasmonic components provide strong electromagnetic enhancement, while the functional materials contribute additional chemical enhancement and improved molecular adsorption. The development of such multifunctional substrates represents a frontier in SERS research, with demonstrated improvements in both sensitivity and specificity for environmental pollutant detection [4] [2].
Comparative Analysis of Enhancement Mechanisms
The relative contributions and characteristics of electromagnetic and chemical enhancement mechanisms have profound implications for SERS substrate design and application. The following diagram illustrates the fundamental processes and synergistic relationship between these two primary enhancement mechanisms in SERS:
The comparative performance of these enhancement mechanisms can be quantitatively evaluated across multiple parameters critical for environmental sensing applications:
Table 3: Direct Comparison of EM and CM Mechanisms
| Parameter | Electromagnetic Enhancement | Chemical Enhancement |
|---|---|---|
| Typical Enhancement Factor | 10ⴠ- 10⸠[1] [2] | 10¹ - 10³ [2] |
| Primary Physical Basis | Plasmon resonance in noble metals | Charge transfer complexes |
| Distance Dependence | Strong (∼dâ»Â¹Â²) [5] | Weak |
| Material Requirements | Au, Ag, Cu nanostructures [2] | Various metals/semiconductors |
| Molecular Specificity | Low | High [3] |
| Optimal Substrate Types | Nanoparticles, nanogaps, 3D architectures [1] | Functionalized surfaces, MXenes [3] |
| Contribution to Total SERS | Major (∼10â¶-10â¸) | Minor (∼10¹-10³) |
| Environmental Application | Broad pollutant detection | Selective target identification |
Experimental Approaches and Methodologies
Protocol 1: Coffee-Ring Effect for Quantitative Dry Analyte Detection
A recent innovative methodology leverages the "coffee ring effect" to improve reproducibility in SERS measurements of transparent dry analytes, particularly relevant for environmental contaminants like glyphosate [6]. This protocol involves adding non-interfering silicon microparticles to the analyte solution, which is then drop-cast onto a silicon-based SERS substrate. During evaporation, the silicon particles aggregate at the drop periphery, concentrating the dry analyte in these defined areas and enabling reproducible laser targeting.
Detailed Methodology:
- Substrate Preparation: Utilize silicon-based SERS substrates with immobilized plasmonic nanoparticles.
- Sample Preparation: Add 1-5 μm silicon microparticles to the analyte solution at optimized concentration.
- Deposition: Apply 10-20 μL of the prepared sample solution onto the substrate surface.
- Drying: Allow controlled evaporation under ambient conditions (23°C, 45% RH recommended).
- Measurement: Focus laser beam at the coffee-ring periphery where analyte concentration is highest.
Performance Data: This approach demonstrated exceptional sensitivity for glyphosate detection with a limit of detection (LOD) of 9.30 × 10â»Â¹â° M and limit of quantification (LOQ) of 9.41 × 10â»Â¹â° M, competitive with established methodologies but without requiring derivatization or extensive sample pretreatment [6]. The method primarily leverages electromagnetic enhancement through the plasmonic substrate while addressing key reproducibility challenges in dry sample analysis.
Protocol 2: Signal-Differentiated SERS Nose Array for Explosive Compound Detection
For detecting environmental contaminants with structural similarities, such as nitro-explosives, a signal-differentiated SERS (SD-SERS) array approach has been developed to enhance discrimination capability [3]. This protocol employs multiple SERS substrates with varied chemical and physical properties to generate differentiated response patterns, enabling more reliable identification through machine learning analysis.
Detailed Methodology:
- Substrate Fabrication: Create six distinct SERS substrates combining:
- Two MXene materials (Mo₂C and Ti₃C₂) for chemical enhancement variation
- Three self-assembled monolayers with different adsorption affinities
- Gold nanobipyramids (AuNBPs) for electromagnetic hot spots
- SD-SERS Array Assembly: Integrate the six substrates into a unified detection platform.
- Sample Exposure: Introduce analyte gas (e.g., TNT) to the array under controlled conditions.
- Spectral Acquisition: Collect Raman spectra from all six substrate elements.
- Pattern Analysis: Process multi-dimensional spectral data with machine learning algorithms (e.g., PCA, LDA) for classification.
Performance Data: Finite-difference time-domain (FDTD) simulations established that AuNBPs provide superior electromagnetic enhancement compared to alternative nanostructures like gold nanostars (AuNSs) or gold nanorods (AuNRs) [3]. The SD-SERS array successfully discriminated TNT from structurally similar compounds (2,4-DNPA) with high accuracy, demonstrating the value of combining multiple enhancement mechanisms for complex environmental detection scenarios.
Protocol 3: Three-Dimensional SERS Substrates for Enhanced Biosensing
Three-dimensional SERS substrates represent a significant advancement over traditional 2D platforms, particularly for analyzing complex biological and environmental samples [1]. These substrates provide volumetric enhancement through increased hot spot density and improved analyte accessibility, achieving enhancement factors exceeding 10⸠with higher reproducibility (RSD typically <10%).
Detailed Methodology:
- Substrate Selection: Choose appropriate 3D architecture based on application:
- Vertically aligned nanowire arrays
- Porous metal frameworks and aerogels
- Dendritic and fractal nanostructures
- Core-shell and hollow nanospheres
- Fabrication Technique: Employ suitable method such as:
- Template-assisted electrochemical deposition
- Galvanic replacement and dealloying
- Freeze-drying and self-assembly processes
- Hybrid integration approaches
- Functionalization: Modify with recognition elements (antibodies, aptamers) for specific targeting.
- Sample Application: Apply liquid samples directly to 3D substrate, leveraging capillary action.
- Signal Acquisition: Utilize standard Raman instrumentation with optimized laser focusing.
Performance Data: 3D SERS substrates consistently outperform 2D equivalents across multiple parameters, offering >10⸠enhancement factors compared to 10âµ-10â· for 2D substrates, with significantly improved reproducibility (RSD <10% vs. moderate reproducibility for 2D) [1]. The 3D architecture facilitates analyte transport and retention in complex matrices like environmental water samples, addressing key limitations of planar substrates.
Research Reagent Solutions for SERS Substrates
The selection of appropriate materials and reagents is fundamental to successful SERS substrate development and application. The following toolkit summarizes essential components and their functions in constructing high-performance SERS platforms for environmental detection:
Table 4: Essential Research Reagent Solutions for SERS Applications
| Reagent Category | Specific Examples | Function in SERS Applications |
|---|---|---|
| Plasmonic Materials | Au, Ag nanoparticles and nanostructures [1] [2] | Provide electromagnetic enhancement via LSPR |
| 2D Materials | Mo₂C MXene, Ti₃C₂ MXene [3] | Enable chemical enhancement through charge transfer |
| Functionalization Agents | Self-assembled monolayers (SAMs), aptamers, antibodies [1] [3] | Enhance selectivity and molecular adsorption |
| Support Structures | Silicon wafers, graphene, porous frameworks [6] [1] | Provide mechanical stability and additional enhancement |
| Shape-Directing Agents | CTAC, CTAB, silver nitrate [3] | Control nanostructure morphology during synthesis |
| Reducing Agents | Sodium borohydride, ascorbic acid, citrate [3] | Facilitate controlled metal nanoparticle growth |
| Additives for Assembly | Silicon microparticles (1-5 μm) [6] | Enable coffee-ring effect for reproducible deposition |
Advanced Substrate Architectures and Performance Trends
The evolution of SERS substrates has progressed from simple metal nanoparticles to sophisticated engineered architectures that optimize both electromagnetic and chemical enhancement mechanisms. Three-dimensional substrates represent a particularly significant advancement, addressing key limitations of traditional 2D platforms through structural innovations that enhance sensitivity, reproducibility, and applicability to complex environmental samples [1].
Table 5: Comparison of 2D vs. 3D SERS Substrates
| Feature | 2D SERS Substrates | 3D SERS Substrates |
|---|---|---|
| Hot Spot Distribution | Confined to planar surface [1] | Volumetric in all dimensions [1] |
| Typical Enhancement Factor | 10âµâ€“10â· [1] | >10⸠[1] |
| Reproducibility | Moderate | High (RSD typically <10%) [1] |
| Analyte Accessibility | Limited surface diffusion [1] | Enhanced via pores and 3D networks [1] |
| Fabrication Methods | Lithography, self-assembly [1] | Template growth, dealloying, freeze-drying [1] |
| Application Flexibility | Limited to flat surfaces | Compatible with irregular surfaces [1] |
The development of multifunctional substrates represents another frontier in SERS technology, particularly for environmental applications [4] [2]. These advanced platforms integrate plasmonic components with additional functional materials such as semiconductors, graphene, metal-organic frameworks (MOFs), and stimuli-responsive polymers. This integration creates synergistic enhancement effects while incorporating capabilities like molecular recognition, preconcentration, and signal modulation [2]. For instance, hydrogel-based SERS substrates with embedded nanoparticles have demonstrated responsive sensing of pH and glucose concentrations in physiological conditions, suggesting potential for adaptive environmental monitoring [1].
The following diagram illustrates the progressive development and classification of SERS substrates, highlighting the evolution from simple metallic structures to advanced multifunctional systems:
Emerging research continues to push the boundaries of SERS performance through innovative substrate designs. Stimuli-responsive architectures that modulate their enhancement properties in response to environmental changes offer promising avenues for smart sensing platforms [1]. Similarly, the integration of digital SERS approaches with artificial intelligence-assisted data processing is addressing traditional challenges in spectral interpretation and quantification, particularly for complex environmental mixtures [5] [4]. These advancements collectively contribute to the growing adoption of SERS beyond specialized research laboratories into practical environmental monitoring applications.
The comparative analysis of electromagnetic and chemical enhancement mechanisms in SERS reveals a complex landscape where substrate design decisions directly impact analytical performance for environmental detection applications. Electromagnetic enhancement provides the dominant contribution to signal amplification, with carefully engineered nanostructures achieving extraordinary enhancement factors exceeding 10⸠through optimized plasmonic properties and hot spot density [1]. Chemical enhancement, while offering more modest amplification, provides valuable molecular specificity and complementary enhancement through charge transfer mechanisms [2] [3].
The evolution toward advanced substrate architectures, particularly three-dimensional and multifunctional platforms, demonstrates the increasing sophistication in harnessing both enhancement mechanisms synergistically [4] [1] [2]. These developments address key challenges in environmental pollutant detection, including sensitivity requirements for trace analytes, reproducibility across complex sample matrices, and selectivity for target compounds in the presence of interferents. The integration of innovative methodological approaches—such as coffee-ring effect utilization, signal-differentiated arrays, and machine learning-assisted analysis—further enhances the practical utility of SERS for environmental monitoring [6] [3].
As SERS technology continues to mature, the deliberate optimization of both electromagnetic and chemical enhancement pathways will remain central to developing next-generation environmental sensors. The ongoing convergence of nanotechnology, materials science, and data analytics promises to overcome current limitations while expanding the application scope of SERS in addressing pressing environmental challenges.
Localized Surface Plasmon Resonance (LSPR) and the Creation of 'Hot Spots'
Surface-Enhanced Raman Scattering (SERS) has emerged as a powerful analytical technique for the ultrasensitive detection of environmental pollutants, leveraging the remarkable signal amplification provided by localized surface plasmon resonance (LSPR) and strategically engineered 'hot spots'. This guide provides a comparative evaluation of SERS substrate technologies, focusing on their LSPR properties and hot spot generation capabilities for pollutant detection. We systematically analyze experimental data and fabrication methodologies for various substrate architectures, highlighting their performance metrics, limitations, and suitability for different environmental monitoring applications. The data presented herein aims to equip researchers with the necessary information to select optimal SERS substrates for specific pollutant detection scenarios.
Localized Surface Plasmon Resonance (LSPR) is a collective oscillation of conduction electrons in metallic nanostructures when excited by incident light at resonant frequencies [2]. This phenomenon generates enhanced localized electromagnetic fields at the nanoparticle surfaces, which dramatically amplify the Raman signals of molecules located near these surfaces—the fundamental basis of Surface-Enhanced Raman Scattering (SERS) [7].
The electromagnetic enhancement mechanism, predominantly responsible for SERS signal amplification (by factors of 10^4-10^8), primarily arises from this LSPR effect when plasmon excitations in metallic nanosystems match the excitation wavelength used for Raman experiments [8]. A secondary chemical enhancement mechanism (typically contributing factors of 10-10^3) involves charge transfer between the plasmonic nanostructures and analyte molecules [7] [2].
'Hot spots' refer to nanoscale gaps (typically <10 nm) between metallic nanostructures where the localized electromagnetic field is significantly enhanced due to plasmon coupling [9]. These regions can provide extraordinary Raman enhancement factors reaching 10^8-10^12, making them crucial for detecting trace-level pollutants in environmental samples [1].
Comparative Performance of SERS Substrates
The design and fabrication of SERS substrates directly influence their LSPR properties, hot spot density, and ultimately, their analytical performance for pollutant detection. The following table compares the key characteristics of major SERS substrate types.
Table 1: Performance Comparison of SERS Substrates for Environmental Pollutant Detection
| Substrate Type | Enhancement Factor (EF) | Hot Spot Characteristics | Reproducibility (RSD) | Representative Pollutants Detected | Limit of Detection (LOD) | Key Advantages | Major Limitations |
|---|---|---|---|---|---|---|---|
| 2D Planar Substrates [1] | 10^5-10^7 | Confined to planar surface; sparse distribution | Moderate (>15%) | Organic dyes, pesticides | ~10^-8 M [8] | Simple fabrication; good for surface characterization | Limited surface area; uneven hot spot distribution |
| 3D Nanostructured Substrates [1] | >10^8 | Volumetric distribution; high density | High (<10%) | Heavy metals, pharmaceuticals [1] | ~10^-12 M [1] | Increased surface area; improved analyte accessibility | Complex fabrication; potential mechanical instability |
| Metal Nanoparticle Colloids [7] | 10^6-10^10 | Dynamic, solution-dependent | Low (~20-30%) | Pesticides, herbicides [8] | 10^-9-10^-15 M [8] | Easy preparation; high enhancement potential | Poor reproducibility; aggregation-dependent |
| Template-Assisted Nanostructures [1] | 10^7-10^9 | Controlled spacing and distribution | Moderate-High (10-15%) | Mycotoxins, organic pollutants [2] | ~10^-10 M | Tunable geometry; relatively scalable | Template removal steps; potential defects |
| Flexible SERS Substrates [10] | 10^6-10^8 | Strain-dependent distribution | Moderate (~15%) | Pesticides on surfaces [2] | ~10^-9 M | Conformal contact; field-deployable | Signal variation with bending; lower enhancement |
The progression from traditional 2D to advanced 3D SERS substrates represents a significant technological evolution. Three-dimensional substrates extend the enhancement volume into the Z-dimension, creating a more isotropic and dense distribution of hot spots compared to their 2D counterparts [1]. Structures such as vertically aligned nanowires, dendritic frameworks, and porous scaffolds generate hot spots throughout their vertical and internal volumes, leading to higher overall enhancement factors exceeding 10^8 and improved signal reproducibility with relative standard deviations typically below 10% [1].
Experimental Protocols for SERS Substrate Evaluation
Protocol 1: Synthesis of Au@Ag Nanocuboids for Dye Detection
Objective: To fabricate a densely packed monolayer of plasmonic Au@Ag nanocuboids for ultrasensitive detection of organic dyes in water samples [8].
Materials:
- Chloroauric acid (HAuClâ‚„) solution
- Silver nitrate (AgNO₃)
- Ascorbic acid (reducing agent)
- Cetyltrimethylammonium bromide (CTAB, surfactant)
- Seed solution (small gold nanoparticles)
Methodology:
- Seed Preparation: Prepare gold nanoparticle seeds by reducing HAuClâ‚„ with sodium borohydride in the presence of CTAB.
- Growth Solution: Prepare a growth solution containing HAuCl₄, AgNO₃, ascorbic acid, and CTAB.
- Nanocuboid Formation: Add seed solution to the growth solution and allow nanocuboids to form over 30 minutes.
- Substrate Assembly: Centrifuge and redisperse nanocuboids in deionized water, then deposit onto a silicon wafer to form a densely packed monolayer.
- Characterization: Use SEM to verify monolayer formation and uniformity.
- SERS Measurement: Apply malachite green (MG) solution (8.7×10^-10 M) in fishpond water to the substrate and acquire SERS spectra with 785 nm excitation.
Results: This substrate achieved a detection limit of 8.7×10^-10 M for MG in fishpond water, with enhancement primarily arising from the edges and corners of nanocuboids that generate numerous electromagnetic hot spots [8].
Protocol 2: Fabrication of TiOâ‚‚/Ag Flower-Like Nanomaterial for Lake Water Monitoring
Objective: To develop a semiconductor-metal hybrid SERS substrate for trace-level pollutant detection in lake waters [8].
Materials:
- Titanium isopropoxide (Ti precursor)
- Silver nitrate (AgNO₃)
- Ethanol and deionized water
- Hydrofluoric acid (HF, morphology control agent)
Methodology:
- TiO₂ Nanostructure Synthesis: Hydrothermally treat titanium isopropoxide with HF at 180°C for 24 hours to form flower-like TiO₂ nanostructures.
- Silver Decoration: Immerse TiO₂ nanostructures in AgNO₃ solution and expose to UV light to photoreduce silver ions to nanoparticles.
- Substrate Characterization: Use SEM/TEM to confirm the uniform distribution of Ag nanoparticles on TiOâ‚‚ surfaces.
- Performance Evaluation: Test the substrate with malachite green solutions in water from Fuxian and Dian lakes with concentrations as low as 10^-12 M.
Results: The TiOâ‚‚/Ag flower-like nanomaterial achieved exceptional detection limits of 10^-12 M for MG in lake waters. The enhancement mechanism combines electromagnetic enhancement from Ag nanoparticle hot spots with chemical enhancement through charge transfer in the molecule-semiconductor-metal system [8].
Fundamental Mechanisms and Theoretical Framework
LSPR and Hot Spot Formation
The following diagram illustrates the fundamental mechanism of LSPR and hot spot formation in metallic nanostructures:
Diagram 1: LSPR and hot spot formation mechanism.
The electromagnetic enhancement in SERS originates from the amplified electromagnetic fields generated when incident light excites LSPR in metallic nanostructures. When plasmonic nanoparticles are closely spaced (typically <10 nm apart), theinteracting electromagnetic fields create localized regions of intense field enhancement known as "hot spots" [9]. In these regions, the Raman signal of molecules can be enhanced by factors up to 10^10-10^12, enabling single-molecule detection in optimal conditions [1].
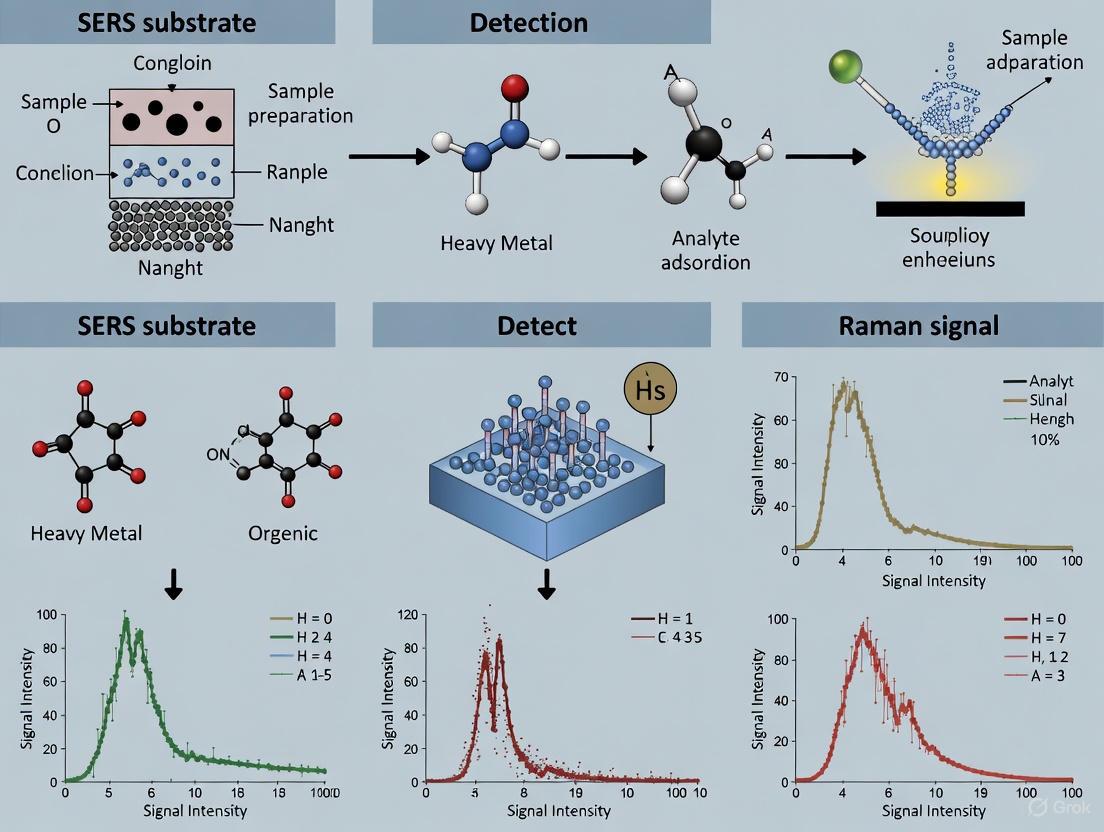
SERS Workflow for Environmental Pollutant Detection
The following diagram outlines a typical SERS-based workflow for detecting pollutants in environmental samples:
Diagram 2: SERS workflow for pollutant detection.
Research Reagent Solutions for SERS Substrate Development
Table 2: Essential Research Reagents for SERS Substrate Fabrication and Application
| Reagent Category | Specific Examples | Function in SERS Technology | Application Notes |
|---|---|---|---|
| Plasmonic Metals | Gold (Au), Silver (Ag), Copper (Cu) nanoparticles | Generate LSPR effect and electromagnetic enhancement | Ag provides highest enhancement but oxidizes; Au offers better stability [2] |
| Shape-Directing Agents | CTAB, citrate, PVP | Control nanostructure morphology and hot spot formation | Critical for creating sharp edges and nanogaps [8] |
| Semiconductor Materials | TiOâ‚‚, ZnO, graphene | Provide chemical enhancement and charge transfer | Used in hybrid substrates for synergistic enhancement [2] |
| Functionalization Agents | Thiols, silanes, antibodies, aptamers | Enable selective capture of target pollutants | Improve specificity in complex environmental matrices [10] |
| Raman Reporters | Rhodamine 6G, crystal violet, thiolated dyes | Serve as signal probes in indirect detection | Must have strong affinity for metal surface and high Raman cross-section [11] |
Advanced Substrate Architectures and Performance Optimization
Recent innovations in SERS substrate design have focused on precisely controlling nanogeometry to maximize hot spot density and LSPR tuning. Three-dimensional SERS substrates represent a significant advancement over traditional 2D platforms, offering volumetric enhancement through architectures such as vertically aligned nanowires, dendritic frameworks, porous scaffolds, and core-shell nanospheres [1].
The electromagnetic enhancement in these advanced structures benefits from multiple factors including light trapping and multiple scattering effects within the 3D matrix, which increase the interaction path length between light and analytes [1]. Furthermore, chemical enhancement can be optimized in hybrid materials that combine plasmonic metals with semiconductors or graphene, creating charge-transfer pathways that additionally amplify Raman signals [2].
For environmental applications, functionalized SERS substrates incorporating molecularly imprinted polymers (MIPs) or specific capture agents like antibodies have demonstrated remarkable selectivity and sensitivity. For instance, a defect-graphene/Ag-MIP substrate achieved an extraordinary detection limit of 2.5×10^-15 M for p-nitroaniline in river water, highlighting the potential of targeted SERS platforms for trace pollutant monitoring [8].
The strategic engineering of LSPR properties and hot spot distribution in SERS substrates has dramatically advanced the capabilities for environmental pollutant detection. As evidenced by the comparative data, 3D substrates and hybrid nanomaterials consistently outperform conventional 2D platforms in terms of enhancement factors, reproducibility, and detection limits for various classes of pollutants.
Future development directions include the creation of stimuli-responsive SERS substrates that modulate their enhancement properties based on environmental conditions, multifunctional hybrid platforms that combine detection with catalytic degradation of pollutants, and data-driven optimization strategies employing machine learning to design optimal nanostructures [1]. The integration of SERS substrates with microfluidic systems for automated sample processing and the development of portable, field-deployable sensors will further expand the practical applications of this powerful technology in environmental monitoring scenarios.
Addressing current challenges related to substrate reproducibility, mechanical stability, and standardization will be crucial for the transition of laboratory-developed SERS substrates to commercially viable environmental sensors. With continued interdisciplinary innovation focusing on both fundamental mechanisms and practical applications, SERS technology is poised to play an increasingly significant role in environmental protection and public health safety.
Surface-Enhanced Raman Scattering (SERS) has emerged as a powerful analytical technique for the sensitive detection of environmental pollutants, leveraging the unique properties of noble metal nanostructures to amplify the weak Raman signals of target molecules. The enhancement primarily arises from two mechanisms: electromagnetic enhancement, driven by localized surface plasmon resonance (LSPR) in noble metals, and chemical enhancement involving charge transfer between the analyte and substrate [12] [1]. Gold (Au), silver (Ag), and their bimetallic nanostructures have become the cornerstone of high-performance SERS substrates due to their exceptional plasmonic properties, tunability, and ability to generate intense electromagnetic "hot spots" [13] [14]. Within environmental monitoring, these substrates demonstrate unparalleled capability in detecting trace-level contaminants such as pesticides, antibiotics, and heavy metals, offering a rapid, non-destructive, and highly specific alternative to conventional analytical methods [15] [16]. This guide provides a comparative evaluation of these noble metal substrates, focusing on their experimental performance in detecting environmental pollutants, to inform researchers and scientists in the field.
Performance Comparison of SERS Substrates
The performance of SERS substrates is quantified by key metrics such as Enhancement Factor (EF), Limit of Detection (LOD), reproducibility, and stability. The table below summarizes the experimental performance of various gold, silver, and bimetallic nanostructures as reported in recent literature.
Table 1: Performance Comparison of Noble Metal-Based SERS Substrates
| Substrate Type | Specific Morphology/Composition | Target Analyte (Application) | Enhancement Factor (EF) | Limit of Detection (LOD) | Key Advantages |
|---|---|---|---|---|---|
| Gold (Au) | Au nanoparticles on Si micro/nano-hybrid structure [13] | Rhodamine 6G (Model compound) | ~10⸠(calculated) | 10â»Â¹Â² M | High sensitivity, excellent stability & reusability |
| Silver (Ag) | Flower-like Ag nanoparticles on flexible sponge [17] | Thiram (Pesticide) | 6.63 × 10ⵠ| 0.1 mg/L | Flexibility, cost-effectiveness |
| Silver (Ag) | Ag nanoparticles self-assembly [18] | Model analyte | Not specified | Near single-molecule | Ultra-high sensitivity |
| Bimetallic (Au-Ag) | MXene-Ni/Ag composite [16] | Thiram (Pesticide) | SPF* of 8.2 × 10ⶠ| 10â»â¹ M | High sensitivity, good reproducibility & stability |
| Bimetallic (Au-Ag) | Au-Ag core-shell nanoparticles [14] | Various food contaminants | 10ⶠto 10¹² (from gaps) | Varies by analyte | Tunable plasmonics, synergistic enhancement |
| Gold (Au) | Laser & plasma-treated AuNPs on glass [19] | Amoxicillin (Antibiotic) | ~3 × 10⸠| 9 × 10â»Â¹â° M | High EF, good consistency, reusability |
*SPF: SERS Performance Factor
Comparative Analysis
- Gold Nanostructures: Substrates like the Au nanoparticle-decorated Si hierarchical structure demonstrate exceptionally high EFs and low LODs for model compounds, alongside remarkable stability and reusability—key for practical applications [13]. The combination of a high-surface-area 3D structure with Au nanoparticles creates abundant hot spots.
- Silver Nanostructures: Silver substrates, such as flower-like Ag particles on sponge, often achieve very high EFs and low LODs, sometimes rivaling or exceeding gold for specific applications [17]. Ag generally provides stronger electromagnetic enhancement than Au but can suffer from oxidation, potentially limiting its long-term stability.
- Bimetallic Nanostructures: Au-Ag bimetallic substrates harness the advantages of both metals. For instance, the MXene-Ni/Ag composite shows high sensitivity for pesticide detection [16]. Au-Ag core-shell and alloy structures exhibit tunable plasmonic properties and synergistic effects that can lead to superior stability and enhancement compared to their monometallic counterparts [14].
Detailed Experimental Protocols
To ensure the reproducibility of SERS-based detection, standardized protocols for substrate fabrication and measurement are crucial. The following sections detail methodologies cited in the performance table.
This protocol describes a method to create a wafer-scale SERS substrate with high sensitivity and stability.
- Step 1: Fabrication of Silicon Micropillars. A P-type <100> silicon wafer is used as the base. Micropillar arrays are fabricated on the silicon surface using photolithography and Inductively Coupled Plasma (ICP) etching.
- Step 2: Catalytic Metal Film Deposition. A thin, continuous film of gold (Au) is deposited onto the micro-structured silicon surface using magnetron sputtering.
- Step 3: Formation of Nanopatterned Au via Thermal Dewetting. The sample is subjected to a thermal annealing process (first dewetting). This causes the thin, metastable Au film to agglomerate into nano-islands and clusters due to surface energy minimization, effectively creating a nanopatterned catalytic mask.
- Step 4: Metal-Assisted Chemical Etching (MACE). The sample undergoes MACE, where the catalytic Au nanopatterns etch the underlying silicon in a solution of HF and Hâ‚‚Oâ‚‚. This forms silicon nanowires on the sidewalls of the micropillars, creating a hierarchical micro/nano-structure.
- Step 5: Decoration with Au Nanoparticles. A second Au film is sputtered onto the hierarchical structure, followed by a second thermal dewetting process. This final step decorates the entire 3D silicon structure with dense, well-adhered Au nanoparticles, which are the primary sources of SERS hot spots.
This protocol outlines the synthesis of a sensitive and reproducible bimetallic composite substrate for trace pesticide detection.
- Step 1: Preparation of MXene Nanosheets. Monolayer Ti₃C₂Tx MXene nanosheets are prepared, typically by selective etching of the Al layer from the MAX phase (Ti₃AlC₂) and subsequent delamination.
- Step 2: Synthesis of Ni/Ag Nanoparticles. Nickel-Silver (Ni/Ag) bimetallic nanoparticles with different atomic ratios are synthesized separately through a chemical reduction method.
- Step 3: Composite Formation via Transmetallation. The pre-synthesized Ni/Ag nanoparticles are modified onto the surface of the monolayer MXene nanosheets. This is achieved through a transmetallation reaction, which facilitates a strong interaction between the nanoparticles and the MXene sheets, forming the final MXene-Ni/Ag composite substrate.
A generalized workflow for acquiring and analyzing SERS spectra from environmental samples is detailed below.
- Step 1: Substrate Preparation. Commercial or lab-fabricated SERS substrates are prepared. They may be used as-is or subjected to pre-cleaning/activation steps (e.g., oxygen plasma treatment) to ensure consistency.
- Step 2: Analyte Adsorption. The target analyte (e.g., pesticide, antibiotic) is adsorbed onto the substrate surface. This is commonly done by depositing a small volume (e.g., 1-10 µL) of the analyte solution onto the substrate and allowing it to dry at room temperature. For some experiments, immersion for a set time (e.g., 1 hour) is used [12].
- Step 3: Raman Spectra Acquisition.
- The substrate is placed under a Raman microscope equipped with a laser source (common wavelengths are 532 nm, 633 nm, or 785 nm).
- The laser power at the sample is set to a non-destructive level (typically a few mW).
- Multiple spectra (e.g., 15-20) are collected from different random spots on the substrate to account for spatial heterogeneity and obtain a statistically representative dataset [12] [20].
- Integration time and number of accumulations are optimized for each substrate-analyte system.
- Step 4: Spectral Preprocessing. Collected spectra are processed to remove cosmic rays, fluorescence background (e.g., using penalized least-squares algorithm), and noise (smoothing). Spectra are often normalized to account for minor fluctuations in laser power or alignment [20].
- Step 5: Data Analysis.
- Quantification: The intensity of a characteristic Raman peak of the analyte is measured and correlated with its concentration using a calibration curve.
- Classification/Screening: Machine learning algorithms (e.g., XGBoost, PCA) can be employed to automatically identify high-quality spectra or classify unknown samples based on their spectral fingerprints [20].
Signaling Pathways and Enhancement Mechanisms
The exceptional performance of SERS substrates is rooted in the fundamental physical and chemical processes that lead to signal amplification. The following diagram and explanation detail these mechanisms.
The overall SERS enhancement is a product of the electromagnetic and chemical mechanisms.
Electromagnetic Enhancement (EM): This is the dominant contributor, accounting for enhancement factors of 10ⶠto 10¹² [1]. When incident laser light strikes a noble metal nanostructure (e.g., a gold nanoparticle), it excites the collective oscillation of conduction electrons, known as Localized Surface Plasmon Resonance (LSPR) [13] [12]. This resonance creates a greatly enhanced electromagnetic field around the nanoparticle. The effect is dramatically amplified in interstitial spaces between particles (nanogaps) or at sharp tips—regions known as "hot spots" [13] [14]. When a target molecule is located within such a hot spot, both the incoming laser light and the outgoing Raman scattered signal are amplified, leading to an enormous boost in the detected Raman intensity.
Chemical Enhancement (CM): This mechanism typically provides a more modest enhancement (10-10³) [1]. It involves a charge transfer process between the energy levels of the metal substrate and the adsorbed analyte molecule. This interaction effectively changes the polarizability of the molecule, leading to an increase in its Raman scattering cross-section. While weaker than the EM effect, chemical enhancement is molecule-specific and contributes to the overall SERS signal.
The Scientist's Toolkit: Essential Research Reagents and Materials
The development and application of high-performance SERS substrates require a suite of specialized materials and reagents. The following table lists key items used in the featured experiments.
Table 2: Essential Research Reagents and Materials for SERS Substrate Development
| Material/Reagent | Function in SERS Research | Example Use Case |
|---|---|---|
| Silicon Wafers | A common, versatile base/support for fabricating structured SERS substrates. | Used as a base for creating micro-pillars and nanowires in hierarchical structures [13]. |
| Gold (III) Chloride Trihydrate (HAuClâ‚„) | A precursor salt for the synthesis of gold nanoparticles (AuNPs) via chemical reduction. | Synthesis of stabilizer-free AuNPs for deposition onto substrates [19]. |
| Silver Nitrate (AgNO₃) | A precursor salt for the synthesis of silver nanoparticles (AgNPs). | Formation of flower-like Ag nanoparticles for flexible sponge substrates [17]. |
| Rhodamine 6G (R6G) / Rhodamine B | Standard dye molecules used as model analytes to evaluate, benchmark, and compare the performance (EF, LOD) of SERS substrates. | Used as a probe molecule to test sensitivity and calculate enhancement factors [13] [12] [19]. |
| Hydrofluoric Acid (HF) | A highly corrosive etchant used in the fabrication of silicon-based nanostructures. | Key component in Metal-Assisted Chemical Etching (MACE) to create silicon nanowires [13]. |
| MXene (Ti₃C₂Tx) | An emerging 2D material used as a support; it concentrates target molecules via strong adsorption, improving sensitivity. | Serves as a platform in the MXene-Ni/Ag composite substrate for pesticide detection [16]. |
| Thiram / Amoxicillin | Representative environmental pollutants (pesticide and antibiotic) used as target analytes to demonstrate real-world application. | Detection of thiram at trace levels to validate substrate performance for food/environmental safety [16] [17]. |
| 9-(Tetrahydrofuran-2-yl)-9H-purine-6-thiol | 9-(Tetrahydrofuran-2-yl)-9H-purine-6-thiol, CAS:42204-09-1, MF:C9H10N4OS, MW:222.27 g/mol | Chemical Reagent |
| 5-Azidoindole | 5-Azidoindole|CAS 81524-74-5|Research Chemical |
Surface-Enhanced Raman Spectroscopy (SERS) has established itself as a powerful analytical technique for the ultrasensitive detection of environmental pollutants, traditionally relying on noble metal substrates like gold and silver nanoparticles for signal amplification. However, the evolution of application requirements—driven by needs for operational durability, cost-effectiveness, and sustainability—has catalyzed the exploration of alternative materials. Emerging non-noble materials, particularly MXenes, graphene oxide, and semiconductor composites, are now challenging the dominance of conventional substrates by offering unique advantages including enhanced stability, tunable surface chemistry, and multifunctionality [21]. These materials leverage sophisticated charge-transfer mechanisms and, when engineered into hybrid structures, can generate synergistic enhancement effects that rival their noble metal counterparts [21] [22]. This guide provides an objective comparison of the SERS performance of these emerging material classes, focusing on their application in detecting environmental pollutants, with supporting experimental data and detailed protocols to inform research and development in this rapidly advancing field.
Performance Comparison of Non-Noble Material Classes
The following tables summarize key performance metrics for the three primary classes of non-noble SERS substrates, based on recent experimental findings.
Table 1: Overall SERS Performance Comparison for Pollutant Detection
| Material Class | Representative Substrate | Target Pollutant | Reported Limit of Detection (LOD) | Enhancement Factor (EF) | Key Advantages |
|---|---|---|---|---|---|
| MXenes | Au NP-engineered Ti3C2Tx | Methylene Blue | 10-11 M [23] | 1010 [23] | Exceptional conductivity, high stability (83% signal after 5 months) [23] |
| Ti3C2Tx (VAF on paper) | Rhodamine B | 20 nM [22] | N/R | Cost-effective, high spot-to-spot reproducibility [22] | |
| Graphene Oxide | N-doped Graphene | Rhodamine B | N/R | 1011 [22] | Strong chemical enhancement (CM) via π-π interactions [24] [22] |
| Semiconductor Composites | Semiconductor/Metal Hybrids | Model Dyes | N/R | 108 - 1011 [21] | Synergistic EM/CM enhancement, photocatalytic self-cleaning [21] |
Table 2: Comparison of Enhancement Mechanisms and Functional Properties
| Material Class | Dominant Enhancement Mechanism(s) | Stability & Recyclability | Remarks / Specific Functionality |
|---|---|---|---|
| MXenes | Chemical (Charge Transfer) [22], can be coupled with EM in hybrids [23] | High; retains 83% signal after 5 months; magnetic composites enable easy recovery [23] [25] | High conductivity promotes efficient charge transfer [22]. Functional groups aid analyte adsorption [26]. |
| Graphene Oxide | Chemical (CM) via charge transfer and π-π interactions [24] [27] | Good; but performance depends on integration with other materials | Excellent for adsorbing aromatic molecules; often used to improve performance of other substrates [24]. |
| Semiconductor Composites | Combined CM and EM (in hybrids) [21] | Excellent; inherent self-cleaning via photodegradation enables substrate reuse [21] | Enables real-time monitoring of photocatalytic reactions and degradation of pollutants [21]. |
Abbreviations: NP (Nanoparticle), VAF (Vacuum-Assisted Filtration), N/R (Not Reported in the reviewed studies), EM (Electromagnetic Enhancement), CM (Chemical Enhancement).
Detailed Experimental Protocols from Key Studies
Protocol: High-Performance SERS Platform Based on Au NP-MXene
- Substrate Synthesis: The substrate was prepared by engineering gold nanoparticles (Au NPs) in situ on Ti3C2Tx MXene nanosheets. This creates a hybrid platform where the MXene provides a robust, conductive foundation and facilitates charge transfer, while the Au NPs contribute electromagnetic enhancement through localized surface plasmon resonance (LSPR) [23].
- SERS Measurement: A Raman spectrometer equipped with a suitable laser source is used. The analyte solution (e.g., Methylene Blue or BDE-47) is drop-cast onto the substrate and allowed to dry. Spectra are then collected from multiple spots to assess signal intensity and reproducibility [23].
- Performance Validation: The platform achieved an exceptional LOD of 10-11 M for Methylene Blue, with an EF of 1010. It also successfully detected the persistent organic pollutant BDE-47 at concentrations below the regulatory threshold of 10-6 M. The relative standard deviation (RSD) was calculated to validate signal repeatability. Stability was confirmed by testing a substrate after 5 months of storage, which retained 83% of its original SERS signal intensity [23].
Protocol: Fabrication of Robust MXene Substrates via Vacuum-Assisted Filtration
- Substrate Fabrication: Few-layer Ti3C2Tx MXene dispersions are prepared. The substrate is created by depositing the MXene dispersion onto filter paper using vacuum-assisted filtration (VAF). This method produces a dense and uniform MXene layer, which is critical for performance [22].
- Comparative Analysis: The study compared VAF against spray coating. VAF resulted in a denser MXene layer with higher uniformity, leading to superior spot-to-spot and substrate-to-substrate reproducibility [22].
- SERS Performance and Analysis: Using Rhodamine B as a probe molecule, the VAF-fabricated substrate demonstrated a low LOD of 20 nM. A key finding was that the surface morphology and the presence of MXene aggregates significantly influence analyte distribution and the resultant SERS enhancement. The dense layer from VAF minimizes unfavorable aggregation, contributing to its superior and reproducible performance [22].
Protocol: Dual-Functional Magnetic Substrate for Detection and Degradation
- Substrate Synthesis: A ternary nanocomposite, MXene@Fe3O4@Ag NPs, was synthesized using co-precipitation and electrostatic self-assembly techniques. Fe3O4 provides magnetism for easy recovery, Ag NPs offer plasmonic enhancement, and MXene serves as the conductive platform [25].
- SERS Detection: The substrate was used for the ultrasensitive detection of Crystal Violet (CV), achieving an extraordinarily low LOD of 1.08 × 10-12 M. Finite-difference time-domain (FDTD) simulations confirmed that the gaps between the Ag NPs on the composite structure create strong electromagnetic "hot spots" responsible for the significant signal enhancement [25].
- Self-Cleaning and Recyclability: Following detection, the same substrate was used for pollutant degradation. Under light irradiation, the composite catalyzes the photo-Fenton reaction, generating hydroxyl radicals that degrade the adsorbed CV molecules. The magnetic property allows the substrate to be easily collected with a magnet after the degradation cycle, enabling its reuse for multiple rounds of detection and degradation [25].
Mechanisms and Workflows: A Visual Guide
The performance of these non-noble materials is governed by distinct enhancement mechanisms. The following diagram illustrates the primary mechanisms and a typical workflow for a dual-functional SERS substrate.
Diagram: SERS enhancement mechanisms and a typical workflow for a detection-degradation-recovery-reuse cycle enabled by advanced composite substrates [21] [25].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagents and Materials for Developing Non-Noble SERS Substrates
| Item | Function / Role | Specific Examples & Notes |
|---|---|---|
| MXene Precursors | Source for synthesizing MXene layers. | MAX phases (e.g., Ti3AlC2); selectively etched to produce Ti3C2Tx [26] [25]. |
| Semiconductor Photocatalysts | Provide chemical enhancement and self-cleaning functionality. | TiO2, ZnO, Fe23; used for charge transfer and photocatalytic degradation of analytes [21]. |
| Graphene Oxide (GO) | Enhances adsorption and chemical enhancement. | GO sheets; improve performance via π-π stacking with aromatic pollutant molecules [24] [21]. |
| Noble Metal Salts | For constructing hybrid substrates with EM enhancement. | Precursors for Ag, Au nanoparticles (e.g., AgNO3); incorporated to create "hot spots" [23] [25]. |
| Magnetic Nanoparticles | Enable substrate recovery and recyclability. | Fe3O4 nanoparticles; allow collection with an external magnet [25]. |
| Cellulose/Paper Substrates | Low-cost, flexible, and sustainable support. | Filter paper or nanocellulose films; serve as a platform for depositing active SERS materials [27] [22]. |
| Model Pollutant Dyes | Standard analytes for evaluating SERS performance. | Rhodamine B (RhB), Methylene Blue (MB), Crystal Violet (CV); used for calibration and LOD determination [23] [22] [25]. |
| Etching Agents | For synthesizing MXenes from MAX phases. | Hydrofluoric Acid (HF) or in-situ HF-forming mixtures; used to selectively remove the 'A' layer from MAX [26]. |
| N,N'-Bis(3-triethoxysilylpropyl)thiourea | N,N'-Bis(3-triethoxysilylpropyl)thiourea Coupling Agent | N,N'-Bis(3-triethoxysilylpropyl)thiourea, a sulfur-functional silane. Used as a coupling agent and for mercury detection. For Research Use Only. Not for human or veterinary use. |
| 2-Hydroxy-3-methoxy-6beta-naltrexol | 2-Hydroxy-3-methoxy-6beta-naltrexol|CAS 57355-35-8 | High-purity 2-Hydroxy-3-methoxy-6beta-naltrexol for analytical research and ANDA development. For Research Use Only. Not for human use. |
The systematic comparison presented in this guide demonstrates that MXenes, graphene oxide, and semiconductor composites are viable and powerful alternatives to traditional noble-metal SERS substrates. MXenes, particularly in hybrid architectures, stand out for their exceptional sensitivity and stability. Semiconductor composites offer the unique advantage of multifunctionality, integrating sensing with self-cleaning via photocatalysis. Graphene oxide plays a crucial role in enhancing analyte adsorption through its rich chemistry.
Future research will likely focus on optimizing the cost-effectiveness and scalability of these materials, especially MXenes, whose long-term stability against oxidation requires further engineering [26]. The integration of biorecognition elements (e.g., aptamers, antibodies) with these substrates is a promising avenue to improve selectivity in complex environmental matrices [24]. As these challenges are addressed, non-noble SERS substrates are poised to become the foundation for the next generation of robust, multifunctional, and field-deployable sensors for environmental monitoring.
Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique that dramatically amplifies the inherently weak Raman scattering signal, enabling single-molecule detection sensitivity [27]. The enhancement mechanism primarily arises from two interconnected phenomena: electromagnetic enhancement (EM) and chemical enhancement (CM). The electromagnetic effect, contributing the majority of signal enhancement (up to 10^8-fold), occurs when localized surface plasmon resonance (LSPR) is excited on nanostructured metal surfaces, generating intensely localized electromagnetic fields known as "hot spots" [28] [8]. The chemical mechanism, while providing more modest enhancement (typically 10-1000-fold), involves charge transfer between the analyte molecules and the substrate surface, which can alter the polarizability of the molecules [29] [28]. The efficiency of both mechanisms is profoundly influenced by the nanoscale morphology of the SERS substrate—specifically the size, shape, and interparticle distance of the nanostructures—which dictates the plasmonic coupling and field confinement effects [30].
For environmental pollutant detection, these morphological parameters determine critical performance metrics including enhancement factor (EF), limit of detection (LOD), and signal reproducibility [8]. This guide systematically compares how different nanostructural characteristics influence SERS performance for detecting trace organic pollutants, heavy metals, and pathogenic microorganisms in environmental samples.
Comparative Analysis of Nanostructural Parameters
The following sections analyze the individual contributions of size, shape, and interparticle distance to SERS enhancement, with quantitative performance data summarized in Table 1.
Nanostructure Size Effects
Nanoparticle size directly governs the spectral position and intensity of the localized surface plasmon resonance. Optimal sizes typically range between 40-160 nm for noble metals, balancing scattering efficiency and field penetration depth [31] [19]. Experimental studies with gold nanodiscs demonstrate that 160nm diameter structures exhibit strong plasmonic resonances in the near-infrared window, which is advantageous for biological and environmental sensing due to reduced background interference [31]. Smaller nanoparticles (10-30 nm) produce weaker electromagnetic fields but higher density coverage, while excessively large structures (>200 nm) support multiple plasmon modes that can broaden the resonance spectrum and reduce enhancement efficiency [30].
Size uniformity critically affects signal reproducibility. Controlled studies using physically synthesized nanodiscs with identical dimensions (160nm diameter, 20nm thickness) revealed that uniform structures provide consistent enhancement factors, whereas polydisperse systems yield unpredictable signal variations [31]. For lead-free halide double perovskite Cs₂AgBiBr₆ nanoflakes, post-growth annealing controlled self-trapped exciton defects, with defect density directly correlating with SERS signal intensity [29].
Nanostructure Shape Effects
Shape determines the curvature and sharpness features where electromagnetic fields concentrate most intensely. Structures with sharp edges, tips, and high aspect ratios—such as nanotriangles, nanocuboids, and nanostars—generate significantly stronger field enhancement compared to spherical nanoparticles due to the lightning rod effect [28] [8].
Comparative studies demonstrate that triangular gold nanoplates assembled with gold nanospheres create double-sided superstructures with abundant hot spots, enabling sensitive detection of pathogenic bacteria like Listeria monocytogenes and S. xylosus [28]. Similarly, Au@Ag nanocuboids arranged in densely packed monolayers leverage their edges and corners to generate intense electromagnetic hot spots, achieving detection of malachite green (MG) at concentrations as low as 10â»Â¹Â² M in lake water [8]. The anisotropic nature of non-spherical structures also enables polarization-dependent SERS responses, which can be exploited for advanced sensing schemes.
Interparticle Distance Effects
Interparticle distance, or "nanogap," is perhaps the most critical parameter for SERS enhancement, with the strongest electromagnetic fields occurring in gaps of 1-10 nm [30]. When nanoparticles are brought within this proximity, their plasmon fields interact synergistically, creating enhancement factors that scale exponentially with decreasing distance [32]. One study established a generalized exponential relationship between SERS efficiency and the non-dimensional interparticle distance/particle diameter ratio for gold and silver nanoisland arrangements [32].
Gradient SERS substrates with systematically varying gap sizes demonstrate this effect clearly, showing that average gap sizes of ~11 nm produce significantly higher enhancement compared to regions with ~50 nm gaps [30]. The formation of connected metal island films with controlled percolation paths represents an effective strategy for creating optimal interparticle separation, as evidenced by annealed gold films that develop interconnected islands with tunable nanogaps [30]. For composite substrates, precise control of nanogaps has been achieved through block copolymer templates that position silver nanoparticles at optimal distances, enabling reproducible hot spot engineering [8].
Table 1: Performance Comparison of SERS Substrates by Morphological Characteristics
| Morphology Characteristic | Substrate Type | Optimal Parameters | Enhancement Factor (EF) | Detection Limit (Pollutant) | Ref. |
|---|---|---|---|---|---|
| Size | Gold nanodiscs | 160 nm diameter, 20 nm thickness | N/A | N/A | [31] |
| Silver nanoparticles | ~45-50 nm diameter | N/A | ~10â»Â¹Â² M (R6G) | [19] | |
| Gold nanoparticles in island film | 10-20 nm radius | N/A | ~10â»â¸ M (BPE/MB) | [30] | |
| Shape | Au@Ag nanocuboids | Sharp edges/corners | N/A | 8.7×10â»Â¹â° M (MG) | [8] |
| Triangular Au nanoplates with Au nanospheres | Double-sided assembly | N/A | Pathogenic bacteria | [28] | |
| Porous gold supraparticles | Interstitial gaps between nanoparticles | N/A | 10â»â¸ M (MGITC) | [8] | |
| Interparticle Distance | Gradient Au island film | ~11 nm gap size | N/A | ~10â»â¸ M (BPE/MB) | [30] |
| Block copolymer with AgNPs | Controlled nanogaps | N/A | 10â»â¶ M (Rhodamine B) | [8] | |
| Lead-free perovskite nanoflakes | Defect-controlled | 5.04×10â· | ~10â»Â¹â° M (MB/R6G) | [29] | |
| Composite Structures | Cold plasma/laser AuNPs | Uniform deposition | ~3×10⸠| 10â»Â¹Â² M (R6G), 9×10â»Â¹â° M (amoxicillin) | [19] |
| Cellulose with metal NPs | Flexible substrate | Up to 10¹¹ | Various pollutants | [27] |
Experimental Protocols for SERS Substrate Fabrication and Evaluation
Fabrication of Gradient SERS Substrates with Multiple Resonances
This protocol describes creating substrates with spatially varying morphology to rapidly screen optimal enhancement parameters [30]:
- Substrate Preparation: Begin with clean glass or silicon substrates. Thermal evaporation deposits a thin gold film (typically 10-30 nm thickness) using a geometry where the evaporation source is positioned at a specific distance from the substrate center to create a thickness gradient.
- Thermal Annealing: Anneal the deposited film under controlled atmosphere (argon or vacuum) at 300-500°C for 30-60 minutes. This process transforms the continuous film into disconnected islands with size and spacing gradients across the substrate.
- Morphological Characterization: Use scanning electron microscopy (SEM) to verify the formation of nanoparticle-like features at substrate edges progressing to connected island structures near the center. Image analysis software (e.g., ImageJ, Gwydion) quantifies particle sizes and gap distributions across different regions.
- Optical Validation: Collect extinction spectra across multiple positions to confirm varying plasmon resonances. The spectral maximum should red-shift and broaden when moving from nanoparticle-rich regions to connected island regions.
Synthesis of Lead-Free Halide Double Perovskite Nanoflakes
This method produces environmentally friendly SERS substrates with enhanced stability [29]:
- Precursor Preparation: Dissolve stoichiometric ratios of cesium (Cs), silver (Ag), and bismuth (Bi) precursors in suitable solvents (typically dimethylformamide or dimethyl sulfoxide).
- Crystallization: Introduce the precursor solution into a poor solvent (typically toluene) under vigorous stirring to induce rapid crystallization of Cs₂AgBiBr₆ nanoflakes.
- Post-Growth Annealing: Anneal the collected nanoflakes under argon atmosphere at 200-300°C for 1-2 hours. This critical step controls self-trapped exciton defects by minimizing AgBi and BiAg anti-site disorders, which directly enhances SERS performance through improved charge transfer.
- Quality Assessment: Characterize crystal structure using X-ray diffraction and analyze photoluminescence spectra to confirm defect density modulation. Higher defect densities correlate with increased SERS signals.
Combined Cold Plasma and Laser Treatment for AuNP Substrates
This rapid fabrication method produces high-performance substrates with excellent reproducibility [19]:
- Substrate Pretreatment: Clean glass slides sequentially with distilled water, ethanol, and acetone. Dry under nitrogen gas. Treat with cold atmospheric pressure plasma (0.9 W power) for 30 seconds to dramatically reduce surface roughness and enhance surface energy (water contact angle decreases from 59° to 0°).
- Nanoparticle Deposition: Apply 3 µL of stabilizer-free gold nanoparticle solution (~45 nm diameter, 1.2 OD concentration) to the confined treatment area.
- Laser-Assisted Assembly: Expose the area to a 532 nm green laser (480 mW power) for 15 minutes. This facilitates uniform deposition of AuNPs across the entire treated area, creating abundant hot spots.
- Performance Validation: Test substrates with rhodamine 6G (R6G) solutions, achieving enhancement factors of ~3×10⸠and detection limits of 10â»Â¹Â² M. The substrates should maintain performance after 10 reuse cycles.
Visualization of SERS Enhancement Mechanisms
The following diagrams illustrate the fundamental relationships between nanostructure morphology and SERS enhancement efficiency.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key Research Reagents and Materials for SERS Substrate Development
| Material/Reagent | Function | Application Example | Key Characteristics |
|---|---|---|---|
| Gold Chloride (HAuCl₄·3Hâ‚‚O) | Gold nanoparticle precursor | Synthesis of AuNPs for SERS substrates [19] | Provides Au³⺠ions for reduction to Auâ°; basis for most gold nanostructures |
| Silver Nitrate (AgNO₃) | Silver nanoparticle precursor | Fabrication of Ag NPs and Ag-based composites [28] | Source of Ag⺠ions; forms high-enhancement silver nanostructures |
| Trisodium Citrate | Reducing and stabilizing agent | Synthesis of spherical Au and Ag nanoparticles [33] | Controls nucleation and growth; prevents aggregation in colloids |
| Lead-Free Perovskite Precursors | Environmentally friendly substrate material | Cs₂AgBiBr₆ nanoflakes for sustainable SERS [29] | Cesium, silver, bismuth salts; avoids lead toxicity while maintaining performance |
| Rhodamine 6G (R6G) | Model analyte for SERS calibration | Evaluation of enhancement factors [29] [19] | Standard dye for performance comparison; well-established Raman fingerprints |
| Methylene Blue (MB) | Model pollutant for detection studies | Trace organic pollutant detection [29] [30] | Cationic dye representing environmental contaminants; used for LOD determination |
| Functionalized Cellulose | Sustainable substrate platform | Flexible, biodegradable SERS substrates [27] | Various forms (nanofibers, crystals); low intrinsic Raman background |
| Block Copolymers (e.g., PS-b-PAA) | Nanostructure template | Controlled assembly of nanoparticles [8] | Creates periodic patterns for precise nanoparticle positioning |
| MgSOâ‚„ | Aggregation agent for colloidal NPs | Salt-induced aggregation for hot spot formation [33] | Induces controlled nanoparticle clustering without competing for surface sites |
| 3-Oxo-2-tetradecyloctadecanoic acid | 3-Oxo-2-tetradecyloctadecanoic Acid | Research-grade 3-Oxo-2-tetradecyloctadecanoic acid for laboratory use. This branched fatty acid is for research use only (RUO). Not for human or veterinary use. | Bench Chemicals |
| 3,4',5-Trihydroxy-3',6,7-trimethoxyflavone | 3,4',5-Trihydroxy-3',6,7-trimethoxyflavone|CAS 578-71-2 | Bench Chemicals |
The systematic comparison presented in this guide demonstrates that precise control over nanostructure morphology—specifically size, shape, and interparticle distance—is fundamental to optimizing SERS substrates for environmental pollutant detection. The quantitative data reveals that enhancement factors spanning from 10â· to 10¹¹ can be achieved through rational morphological design, enabling detection limits as low as 10â»Â¹âµ M for certain pollutants [29] [27] [8].
Future developments in SERS substrate technology will likely focus on multifunctional morphologies that combine optimal geometrical parameters with advanced material properties. Lead-free perovskite nanoflakes represent a promising direction, addressing toxicity concerns while maintaining high enhancement factors through defect engineering [29]. Similarly, sustainable substrates based on functionalized cellulose offer an environmentally responsible alternative without compromising performance [27]. The integration of machine learning approaches with high-throughput fabrication methods, such as gradient substrates, will accelerate the discovery of optimal morphological parameters for specific environmental applications [30]. As standardization in enhancement factor calculation methodologies improves, more reliable comparisons between different morphological strategies will emerge, further advancing the field of SERS-based environmental monitoring [27].
SERS in Action: Deploying Advanced Substrates for Real-World Pollutant Detection
Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique for the detection of trace-level environmental pollutants, combining the molecular fingerprinting capability of Raman spectroscopy with significant signal amplification. This guide focuses on the evaluation of SERS substrates for detecting organophosphorus and carbamate pesticides at sub-μg L−1 levels, a critical requirement for environmental and food safety monitoring. The core principle of SERS relies on the dramatic enhancement of Raman signals when target molecules are adsorbed onto or near specially designed nanostructured surfaces, primarily through electromagnetic and chemical enhancement mechanisms [7]. The development of reliable SERS substrates has become a central research theme in environmental pollutant detection, with ongoing efforts to improve sensitivity, reproducibility, and applicability to real-world samples [34].
Performance Comparison of SERS Substrates
The analytical performance of SERS substrates varies significantly based on their material composition, nanostructure design, and functionalization strategies. The tables below summarize key performance metrics for various substrate types reported in recent literature.
Table 1: Performance comparison of SERS substrates for pesticide detection
| Substrate Type | Target Pesticides | Detection Limit | Enhancement Factor | Reproducibility (RSD) | Key Advantages |
|---|---|---|---|---|---|
| Noble metal nanoparticles (Ag/Au) [35] [7] | Organophosphorus pesticides | sub-μg L−1 to low μg L−1 | Up to 10^10 [7] | 5-15% [35] | High enhancement, tunable plasmonics |
| Bimetallic hybrids [35] | Organophosphorus compounds | Sub-μg L−1 range | Not specified | 5-15% | Improved stability and sensitivity |
| MOF-derived systems [35] | Various pesticides | Low μg L−1 | Not specified | Not specified | High surface area, selective adsorption |
| Hydrogel-loaded Ag nanoparticle aggregates [36] | Model pollutants (Malachite Green) | 10^-12 M for Nile Blue [36] | 1.4 × 10^7 for MG [36] | 6.74% (200 μm × 200 μm area) [36] | Salt-resistant, 3D hot spots |
| Semiconductor-photocatalyst hybrids [37] | Organic molecules | Varies by target | Not specified | Not specified | Self-cleaning, reusable |
Table 2: SERS detection of specific pesticide classes
| Pesticide Class | Characteristic Functional Groups & Vibrational Bands | Representative Substrates | Typical Detection Limits | Matrix Applications |
|---|---|---|---|---|
| Organophosphorus [35] [38] | P=O, P=S groups ~650-850 cmâ»Â¹ [35] | Au/Ag nanostructures, aptamer-functionalized substrates [35] [39] | sub-μg L−1 to low μg L−1 [35] | Fruit/vegetable surfaces, juices, grains, water [35] |
| Carbamates [38] | C-N stretching ~1000-1100 cmâ»Â¹ [38] | Functionalized noble metal nanoparticles | Not specified | Tomato peels, agricultural products [38] |
| Pyrethroids [38] | Benzene ring breathing modes, C=C stretching [38] | Portable Raman systems with 1064 nm laser | Not specified | Tomato peels, crop surfaces [38] |
Experimental Protocols for SERS Substrate Evaluation
Substrate Fabrication and Characterization
Hydrogel-Loaded Silver Nanoparticle Aggregates (3D-SERS) This innovative substrate combines physically induced colloidal silver nanoparticle aggregates (AgNAs) with an agarose hydrogel matrix to create a three-dimensional SERS-active material [36]. The fabrication begins with synthesis of monodisperse silver nanoparticles (AgNPs) by heating deionized water (250 mL) containing glycerol (1 mL) to 95°C, followed by addition of silver nitrate (45 mg) and sodium citrate (5 mL, 1%) under vigorous stirring [36]. After continuous heating for 30 minutes until the solution turns greenish brown, the AgNPs are cooled, concentrated tenfold, and subjected to a freeze-thaw process (-20°C for 12 hours, then thawed at room temperature) [36]. The thawed dispersion is sonicated for 10 minutes to form AgNAs. For hydrogel incorporation, 1 mL of 2% agarose solution is mixed with 100 μL of 10-fold-concentrated AgNAs solution, heated to 90°C until homogenized, then rapidly cooled in a Petri dish to form the final 3D substrate [36].
Commercial Gold Nanostructured Substrates Comparative studies often include commercially available SERS substrates. For instance, Type A substrates feature glass covered with gold nanostructures, Type B consists of silicon plates with gold nanostructures, and Type C contains gold and silver nanostructures on silicon [12]. These substrates are characterized using scanning electron microscopy (SEM) to analyze surface morphology, particle size distribution, and interstructural distances, which critically influence SERS enhancement [12].
Analyte Preparation and Measurement Procedures
Standard Solution Preparation For quantitative evaluation, Rhodamine B solutions are typically prepared across concentrations ranging from 10^-2 M down to 10^-12 M [12]. The base 10^-2 M solution is prepared by mixing 0.144 g of Rhodamine B with 30 mL of deionized water, followed by serial tenfold dilutions [12]. For pesticide detection, stock solutions are prepared in appropriate solvents (e.g., methanol or deionized water) at concentrations of 50% v/v, then applied to real-world samples like tomato peels for validation studies [38].
SERS Measurement Protocol Substrates are immersed in analyte solutions for predetermined times (e.g., 1 hour for Rhodamine B), then removed and dried for 15 minutes before measurement to increase analyte proximity to active surfaces and quench fluorescence [12]. Measurements are typically performed using Raman spectrometers with 532 nm excitation lasers, though 1064 nm lasers are increasingly used to reduce fluorescence in biological samples [38]. The system is calibrated using a crystalline silicon plate (520 cm^-1 peak) before measurements [12]. Multiple measurements (15-20 points) are averaged to minimize signal fluctuations, with fluorescence backgrounds removed using spline approximations [12].
Enhancement Factor Calculation The analytical enhancement factor (AEF) is calculated using the formula: AEF = (ISERS / IRaman) × (CRaman / CSERS) where ISERS and IRaman are the measured intensities of a specific Raman peak in SERS and normal Raman measurements, respectively, and CRaman and CSERS are the corresponding analyte concentrations [12].
Signaling Pathways and Workflows
The SERS detection process involves multiple interconnected steps from substrate design to analyte detection. The following diagram illustrates the complete workflow for SERS-based pesticide detection.
SERS Detection Workflow for Pesticide Analysis
The SERS enhancement mechanism involves two primary pathways that operate synergistically to amplify Raman signals, as illustrated in the following diagram.
SERS Enhancement Mechanisms
Research Reagent Solutions
Successful SERS-based pesticide detection requires carefully selected materials and reagents optimized for specific detection scenarios.
Table 3: Essential research reagents for SERS-based pesticide detection
| Reagent/Material | Function/Purpose | Example Applications | Key Considerations |
|---|---|---|---|
| Silver nanoparticles (AgNPs) [12] [36] | Primary SERS substrate, electromagnetic enhancement | Environmental pollutant detection, pesticide monitoring [34] [36] | Size (20-100 nm), shape, aggregation control |
| Gold nanoparticles (AuNPs) [12] | SERS substrate, better chemical stability than Ag | Commercial substrates, bio-sensing [12] | Size, surface chemistry, functionalization |
| Sodium citrate [36] | Reducing and stabilizing agent for nanoparticle synthesis | AgNP synthesis [36] | Concentration affects particle size and distribution |
| Agarose hydrogel [36] | 3D substrate matrix, prevents nanoparticle aggregation | Salt-resistant substrates for environmental monitoring [36] | Concentration, pore size, loading capacity |
| Antibodies & aptamers [39] | Bio-recognition elements for selective pesticide capture | SERS biosensors for specific pesticide detection [39] | Specificity, affinity, stability |
| Rhodamine B [12] | Model compound for substrate evaluation & calibration | Enhancement factor calculation [12] | Concentration range, fluorescence quenching |
| Metal-organic frameworks (MOFs) [34] [35] | Porous materials for analyte preconcentration | Selective pesticide capture [34] | Pore size, surface functionality |
| Molecularly imprinted polymers (MIPs) [34] | Synthetic receptors for selective binding | Pesticide detection in complex matrices [34] | Template selection, binding affinity |
The detection of organophosphorus and carbamate pesticides at sub-μg L−1 levels represents a significant challenge in environmental monitoring, with SERS technology offering a promising solution through its exceptional sensitivity and molecular specificity. Current research demonstrates that carefully engineered SERS substrates, including noble metal nanostructures, bimetallic hybrids, and innovative 3D hydrogel composites, can achieve detection limits in the sub-μg L−1 to low μg L−1 range with reproducibility of 5-15% RSD under optimized conditions [35] [36]. The integration of biological recognition elements such as antibodies and aptamers further enhances selectivity in complex matrices [39]. Future developments in standardized fabrication protocols, portable detection systems, and computation-guided substrate designs will accelerate the translation of SERS technology from laboratory research to practical environmental surveillance applications [35]. As substrate engineering continues to evolve with emerging materials and improved understanding of enhancement mechanisms, SERS is poised to become an increasingly powerful tool for ensuring environmental safety and protecting public health from pesticide contamination.
Surface-Enhanced Raman Scattering (SERS) has emerged as a powerful analytical technique for the detection of environmental pollutants, offering exceptional sensitivity, molecular fingerprinting capability, and potential for field deployment. Within the broader context of evaluating SERS substrates for environmental pollutant detection, this guide focuses specifically on the detection of heavy metal ions—lead (Pb²âº), mercury (Hg²âº), and uranium (UO₂²âº)—which present significant environmental and health risks even at trace concentrations. The detection of these metal ions poses unique challenges as they typically do not exhibit intrinsic Raman activity and often require sophisticated substrate engineering to achieve selective and sensitive detection. This comparison guide objectively evaluates the performance of various SERS-based strategies, substrates, and recognition elements for these three priority metal ions, providing researchers with critical experimental data and methodologies to inform substrate selection and protocol development.
SERS Enhancement Mechanisms and Substrate Design
The remarkable sensitivity of SERS stems primarily from two enhancement mechanisms: electromagnetic enhancement (EM) and chemical enhancement (CM). EM arises from the localized surface plasmon resonance (LSPR) effect occurring at rough noble metal surfaces or nanostructures, generating intensely localized electromagnetic fields known as "hot spots" that can enhance Raman signals by factors of 10â¶-10⸠[2]. CM involves charge transfer between the analyte molecules and the substrate surface, typically contributing enhancement factors of 10-100 [40] [2]. In practical SERS applications for metal ion sensing, both mechanisms often operate synergistically.
Substrate architecture plays a crucial role in determining SERS performance. Traditional two-dimensional (2D) substrates provide limited hot spot density and analyte accessibility, while three-dimensional (3D) substrates—including vertically aligned nanowires, dendritic nanostructures, porous frameworks, and hierarchical hybrid structures—offer enhanced sensitivity through volumetric hot spot distribution and improved molecular diffusion [1]. The design of 3D substrates extends the enhancement volume into the z-axis, creating more dense and reproducible hot spots that significantly boost detection capabilities for trace metal ions [1].
Table 1: Comparison of 2D vs. 3D SERS Substrates for Metal Ion Detection
| Feature | 2D SERS Substrates | 3D SERS Substrates |
|---|---|---|
| Hot Spot Distribution | Confined to planar surface | Distributed volumetrically |
| Enhancement Factor | 10âµâ€“10â· | >10⸠|
| Reproducibility | Moderate | High (RSD typically < 10%) |
| Analyte Accessibility | Limited diffusion on surface | Enhanced diffusion via pores and 3D networks |
| Fabrication Methods | Lithography, self-assembly | Template growth, dealloying, freeze-drying |
Detection Strategies for Heavy Metal Ions
Direct vs. Indirect Detection Approaches
Heavy metal ion detection via SERS primarily employs two fundamental strategies. Direct detection relies on the inherent affinity between the metal ion and a functionalized substrate surface, where the formation of a surface complex generates a measurable SERS signal [41]. This approach is relatively straightforward but is generally limited to metal ions that form strong complexes with Raman-active ligands on the substrate surface.
Indirect detection dominates SERS-based metal ion sensing, particularly for ions with weak Raman cross-sections [42] [43]. This approach utilizes molecular recognition elements (MREs)—such as DNAzymes, aptamers, antibodies, or specific organic ligands—that undergo conformational changes or reactivity upon binding target metal ions. These changes are transduced into measurable SERS signals through labeled Raman reporters or substrate modification. Indirect strategies offer superior selectivity and sensitivity, enabling detection limits that surpass conventional analytical methods for heavy metal ions [42] [41].
Signaling Mechanisms and Recognition Chemistry
The selectivity of SERS detection for specific metal ions is achieved through carefully designed recognition chemistry. For mercury ions (Hg²âº), the strong and specific thymine-Hg²âº-thymine (T-Hg²âº-T) base pairing in DNA structures provides exceptional selectivity [42] [41]. This interaction facilitates the formation of hairpin DNA structures that bring Raman reporters into proximity with the SERS substrate, generating enhanced signals. Alternative approaches exploit the direct amalgamation reaction between Hg²⺠and silver or gold substrates, leading to measurable signal attenuation of pre-adsorbed Raman reporters [42].
For lead ions (Pb²âº), DNAzyme-based sensors represent the predominant strategy [41]. These catalytic DNA molecules cleave specific substrates in the presence of Pb²âº, resulting in distance changes between nanoparticles or the release of Raman reporters that generate distinct SERS signals. The high binding specificity of DNAzymes to Pb²⺠enables exceptional selectivity in complex environmental matrices.
Uranyl ions (UO₂²âº) detection leverages the strong complexation with specific organic ligands or the high adsorption capacity of advanced materials such as covalent organic frameworks (COFs) [44] [41]. The COF TpPa-1, for instance, exhibits a remarkable maximum adsorption capacity of 1194.07 mg UO₂²⺠per gram of material, enabling exceptional pre-concentration and detection capabilities [44]. The subsequent formation of Ag/Agâ‚‚O nanoparticles on the UO₂²âº-loaded COF creates abundant hot spots for SERS signal enhancement.
Figure 1: SERS Detection Strategies for Heavy Metal Ions. The diagram illustrates both direct detection (via surface complex formation) and the more common indirect detection approach using recognition elements and Raman reporters.
Comparative Performance Analysis
Quantitative Detection Capabilities
Recent advances in SERS substrate engineering and recognition chemistry have achieved exceptional detection sensitivity for heavy metal ions, often surpassing regulatory requirements for environmental monitoring. The following table summarizes the performance characteristics of state-of-the-art SERS strategies for lead, mercury, and uranium detection.
Table 2: Performance Comparison of SERS Detection Methods for Heavy Metal Ions
| Metal Ion | SERS Substrate/Strategy | Linear Detection Range | Limit of Detection (LOD) | Selectivity Characteristics |
|---|---|---|---|---|
| Uranium (UO₂²âº) | Ag/Agâ‚‚O–COF TpPa-1 composite | 10â»â¸ to 10â»â¶ mol/L | 8.9 × 10â»Â¹â° mol/L | Excellent selectivity against other metal ions and oxo-ions [44] |
| Mercury (Hg²âº) | AgNPs@tapered optical fiber probe | 10â»Â¹Â² to 10â»â´ mol/L | 5.15 × 10â»Â¹Â³ mol/L | Good selectivity against Ca²âº, Al³âº, Fe²âº, Mg²âº, Zn²âº, Ba²âº, Cu²âº, Pb²⺠[42] |
| Mercury (Hg²âº) | DNA-based thymine-Hg²âº-thymine complex | - | 1 × 10â»Â¹Â² mol/L (0.2 ppt) | Exceptional specificity due to DNA structural transition [41] |
| Lead (Pb²âº) | DNAzyme-based SERS biosensor | - | - | High binding specificity for Pb²⺠ions [41] |
| Mercury (Hg²âº) | Tryptophan-modified Au nanomaterials | - | 5 ppb | Selective complex formation with Hg²⺠[41] |
Substrate Materials and Functionalization
The selection of substrate materials and their functionalization strategies critically determines the sensitivity, selectivity, and practical applicability of SERS-based heavy metal ion detection. Noble metals—particularly silver and gold—remain the predominant materials due to their strong plasmonic properties in the visible spectrum [45]. However, emerging hybrid materials that combine noble metals with functional nanomaterials offer enhanced capabilities through synergistic effects.
Silver-based substrates generally provide higher enhancement factors than gold but are more susceptible to oxidation, which can compromise long-term stability [45]. Gold substrates offer superior chemical stability and easier functionalization with thiolated recognition elements, making them preferable for complex environmental samples [45]. The integration of covalent organic frameworks (COFs) with traditional plasmonic materials, as demonstrated in uranium detection, provides both exceptional adsorption capacity and SERS enhancement [44]. Paper-based substrates have gained attention for field deployment due to their low cost, flexibility, and capacity for creating concentrated analyte zones through capillary action [46] [27].
Table 3: SERS Substrate Materials and Their Properties for Metal Ion Detection
| Substrate Material | Key Advantages | Limitations | Representative Applications |
|---|---|---|---|
| Silver Nanoparticles (AgNPs) | High enhancement factor, strong plasmonic response | Susceptible to oxidation, moderate stability | Hg²⺠detection on tapered optical fiber [42] |
| Gold Nanoparticles (AuNPs) | Excellent stability, easy functionalization, biocompatibility | Lower enhancement than silver, higher cost | DNAzyme-based Pb²⺠detection [41] |
| Ag/Ag₂O–COF Composite | High adsorption capacity, synergistic enhancement, selectivity | Complex fabrication, potential pore blocking | Ultrasensitive UO₂²⺠detection [44] |
| Paper-based Substrates | Low cost, flexibility, concentrative ability | Lower enhancement, potential background interference | Pesticide and heavy metal detection [46] |
| Non-noble Metal Substrates | Cost-effective, adjustable band structure, complementary selectivity | Generally lower enhancement factors | Emerging alternative for specific applications [40] |
Experimental Protocols and Methodologies
Protocol for Uranyl Ion Detection Using Ag/Ag₂O–COF Composite
Substrate Preparation [44]:
- COF TpPa-1 Synthesis: Prepare TpPa-1 via hydrothermal method by reacting 1,3,5-triformylphloroglucinol (Tp) and 1,4-diaminobenzene (Pa-1) in a mixed solvent system. Characterize the product using SEM, XRD, and FT-IR to verify crystalline structure and functional groups.
- Uranyl Enrichment: Immerse the COF TpPa-1 in the sample solution containing uranyl ions for predetermined time to allow efficient adsorption.
- Ag/Ag₂O Nanoparticle Formation: Dope the uranyl-loaded COF with AgNO₃ solution in an optimal ratio. Transform silver ions into Ag/Ag₂O composite nanoparticles under Raman laser irradiation to create the active SERS substrate.
SERS Measurement [44]:
- Instrument Parameters: Utilize a Raman spectrometer with 785 nm excitation wavelength, approximately 5 mW laser power at the sample, and 20s integration time.
- Spectral Analysis: Identify the characteristic Raman shift for uranyl ions around 830 cmâ»Â¹, corresponding to the symmetric O=U=O stretching vibration.
- Quantification: Construct calibration curves using standard solutions across the concentration range of 10â»â¸ to 10â»â¶ mol/L.
Validation [44]:
- Verify method accuracy through comparison with ICP-MS results.
- Test selectivity by challenging with potential interfering ions including Cu²âº, Pb²âº, Zn²âº, Cd²âº, Cr³âº, Fe³âº, Naâº, Kâº, Ca²âº, Mg²âº, CO₃²â», SO₄²â», and PO₄³â».
Protocol for Mercury Ion Detection Using Tapered Optical Fiber Probe
Substrate Fabrication [42]:
- Tapered Optical Fiber Preparation: Fabricate tapered optical fiber (TOF) tip using heating and stretching method to achieve optimal cone angle for SERS sensitivity.
- AgNPs Modification: Decorate the TOF tip surface with silver nanoparticles via chemical deposition using hydroxylamine hydrochloride as reducing agent.
- Characterization: Verify AgNPs modification and distribution using SEM imaging.
SERS Detection Procedure [42]:
- Baseline Measurement: Immerse the AgNPs@TOF probe in Rhodamine 6G (R6G) solution (10â»â¶ M) as Raman reporter and acquire initial SERS spectrum.
- Sample Introduction: Add Hg²⺠standard or sample solution to the R6G solution and initiate reaction timer.
- Kinetic Monitoring: Record SERS spectra at 1-minute intervals for 5 minutes total reaction time.
- Signal Analysis: Monitor attenuation rate of characteristic R6G peak at 1360 cmâ»Â¹. Calculate rate of signal decrease, which correlates with Hg²⺠concentration.
Calibration [42]:
- Establish calibration curve using Hg²⺠standard solutions across 10â»Â¹Â² to 10â»â´ M concentration range.
- Determine LOD based on signal-to-noise ratio of 3 for the lowest detectable concentration.
General Protocol for DNA-Based Heavy Metal Ion Detection
Probe Design [41]:
- Recognition Element Selection: Design thymine-rich DNA strands for Hg²⺠detection or DNAzyme systems for Pb²⺠detection.
- Substrate Functionalization: Immobilize thiolated DNA probes on gold nanoparticles or substrates via gold-thiol self-assembled monolayer formation.
- Raman Reporter Attachment: Label complementary DNA strands with Raman-active molecules (e.g., Cy5, 4-NTP) for indirect detection.
Assay Procedure [41]:
- Sample Incubation: Expose functionalized substrate to sample solution containing target metal ions for optimal binding duration (typically 30-60 minutes).
- Signal Generation: For structure-switching sensors, allow formation of T-Hg²âº-T complexes or DNAzyme cleavage to alter distance between nanoparticles and Raman reporters.
- SERS Measurement: Acquire spectra using appropriate laser excitation (varies with reporter molecule).
- Data Analysis: Quantify metal ion concentration based on intensity changes at reporter-specific Raman shifts.
Figure 2: Experimental Workflow for SERS-Based Heavy Metal Ion Detection. The flowchart outlines the key steps in sample processing, substrate preparation, and measurement, highlighting decision points for method selection.
Essential Research Reagents and Materials
The successful development and implementation of SERS-based heavy metal ion detection requires specific research reagents and functional materials. The following table summarizes essential components and their roles in creating effective SERS sensing platforms.
Table 4: Essential Research Reagents for SERS-Based Heavy Metal Ion Detection
| Reagent/Material | Function/Application | Examples/Specifications |
|---|---|---|
| Silver Nitrate (AgNO₃) | Precursor for silver nanoparticle synthesis | 99.8% purity; used for substrate fabrication [44] [42] |
| Gold Nanoparticles | Plasmonic substrate material | Various shapes: nanospheres, nanotriangles, nanostars; different sizes (20-100 nm) [45] |
| Covalent Organic Frameworks (COFs) | Adsorption and pre-concentration of target ions | COF TpPa-1 for UO₂²⺠with high adsorption capacity (1194.07 mg/g) [44] |
| DNA Probes/Aptamers | Recognition elements for selective binding | Thymine-rich DNA for Hg²âº; DNAzymes for Pb²⺠[41] |
| Raman Reporters | Signal generation in indirect detection | Rhodamine 6G, 4-mercaptobenzoic acid (MBA), 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB) [42] [43] |
| Functionalized Cellulose | Low-cost, flexible substrate platform | Filter paper loaded with AgNPs and ZnO for pesticide and metal detection [46] [27] |
| Hydroxylamine Hydrochloride | Reducing agent for silver nanoparticle synthesis | Used for AgNPs decoration on tapered optical fiber [42] |
SERS technology has demonstrated exceptional capabilities for detecting heavy metal ions at environmentally relevant concentrations, with each target metal requiring specialized substrate designs and recognition strategies. Uranium detection benefits tremendously from hybrid materials like Ag/Ag₂O–COF composites that combine high adsorption capacity with plasmonic enhancement. Mercury detection achieves remarkable sensitivity through both DNA-based recognition and amalgamation reactions with silver substrates. Lead detection leverages the specificity of DNAzyme systems for selective identification in complex matrices.
Future developments in SERS-based heavy metal ion detection will likely focus on creating multifunctional composite substrates with improved stability and reproducibility, integrating artificial intelligence for spectral analysis and quantification, developing portable and field-deployable platforms for on-site monitoring, and establishing standardized protocols for inter-laboratory reproducibility. As substrate engineering and recognition chemistry continue to advance, SERS technology is poised to become an indispensable tool for environmental monitoring, food safety testing, and biomedical analysis of heavy metal contaminants.
Analysis of Marine Biotoxins and Mycotoxins in Complex Matrices
Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a transformative analytical technique for detecting trace-level environmental pollutants, particularly marine biotoxins and mycotoxins in complex matrices. This review evaluates advanced SERS substrates against traditional analytical methods, framing the comparison within the broader thesis that engineered nanostructures significantly enhance detection capabilities for environmental monitoring. Unlike conventional chromatography and immunoassays, SERS leverages plasmonic effects in metallic nanostructures to amplify inherently weak Raman signals by factors of 10ⶠto 10¹â´, enabling single-molecule detection sensitivity in ideal conditions [2] [47]. The technology's unique advantages—including molecular fingerprint specificity, minimal sample preparation, resistance to water interference, and compatibility with portable instrumentation—position it as a core solution for on-site rapid detection of biotoxins that threaten food safety and public health [48] [49].
Mycotoxins (e.g., aflatoxins, ochratoxins) and marine biotoxins (e.g., saxitoxin, okadaic acid) represent particularly challenging analytes due to their low molecular weights, low concentration in complex samples, and severe health implications [48] [47]. The European Food Safety Authority reported 287 shellfish toxin contamination incidents in the EU in 2022 alone, with saxitoxin causing 62% of these cases and triggering emergency recalls of 43,000 metric tons of seafood products [47]. Similarly, mycotoxins affect approximately 25% of global crops, causing massive economic losses and chronic health risks including gene mutation, cancer, and organ damage [50]. This review systematically compares SERS substrate technologies through the lens of their operational principles, analytical performance, and practical applicability for detecting these hazardous compounds in real-world environments.
Comparative Analysis of SERS Substrates and Traditional Methods
Performance Comparison Table
Table 1: Comparative analysis of SERS substrates and traditional methods for biotoxin detection
| Technology | Enhancement Mechanism | Detection Limit | Analysis Time | Multiplexing Capability | Portability | Key Applications |
|---|---|---|---|---|---|---|
| Colloidal SERS Substrates | LSPR, "hot spots" from nanoparticle aggregation [48] | ~ppm-ppb [48] | Minutes [48] | Moderate (label-free and spatial encoding) [51] | High (portable Raman systems) [48] | Preliminary screening, mycotoxin detection [48] |
| 2D Solid SERS Substrates | Electromagnetic enhancement on planar surfaces [1] | ~ppb-ppt [1] | 15-30 minutes [1] | Moderate (limited by surface area) [1] | Moderate to High [1] | Laboratory analysis, fundamental studies [1] |
| 3D SERS Substrates | Volumetric "hot spots," multiple scattering effects [1] | >ppb-ppt (EF > 10â¸) [1] | 10-20 minutes [1] | High (increased binding sites) [1] | Moderate (some require benchtop systems) [1] | Complex matrices, low-abundance toxins [1] |
| Ag-pSi Microarray | Silver-coated porous silicon with optimized pore morphology [50] | 0.008-0.922 ppb for mycotoxins [50] | ~90 minutes including biorecognition [50] | High (25-spot microarray) [50] | High (validated with portable Raman) [50] | Multi-analyte mycotoxin screening in foodstuffs [50] |
| Cellulose-Based SERS | Functionalized with metal NPs on flexible cellulose [27] | EF up to 10¹¹ [27] | Minutes [27] | Moderate (flexible, adaptable format) [27] | High (flexible, lightweight) [27] | Field detection, irregular surfaces [27] |
| HPLC/HPLC-MS | Chromatographic separation, mass detection [48] [47] | ppt-ppb [48] | Hours to days [48] | Low to Moderate [48] | Low (laboratory-bound) [48] | Regulatory compliance, reference methods [48] |
| ELISA/GICA | Antibody-antigen binding with enzymatic/colorimetric detection [48] [47] | ppb [48] | 1-2 hours [48] | Low (typically single-analyte) [48] | Moderate (kits for on-site use) [48] | Rapid screening, field testing [48] |
Key Advantages of SERS Technology
SERS technology demonstrates distinct advantages across multiple performance dimensions compared to traditional detection methods. The technique achieves exceptional sensitivity through localized surface plasmon resonance (LSPR), where incident light excites collective oscillations of conduction electrons in noble metal nanostructures, creating enhanced electromagnetic fields at "hot spot" regions [2]. This physical amplification mechanism enables SERS to overcome the inherent weak signal intensity of conventional Raman spectroscopy while providing molecular fingerprint information unavailable from immunoassays [48] [51]. The fingerprinting capability allows simultaneous detection of multiple contaminants through either label-free approaches (directly targeting intrinsic molecular vibrations) or labeled methods using Raman reporter molecules with distinct spectral signatures [51].
The operational practicality of SERS further distinguishes it from traditional methods. Sample preparation is significantly simplified compared to chromatographic techniques, with minimal requirements for extraction and purification [49]. Analysis times range from minutes for simple colloidal substrates to approximately 90 minutes for sophisticated aptamer-functionalized microarrays, substantially faster than the hours to days required for HPLC-MS analysis [48] [50]. Perhaps most significantly, SERS compatibility with portable Raman instrumentation enables field-deployable quantification of biotoxins at relevant contamination levels, addressing critical needs for on-site monitoring in food production, agricultural, and marine environments [48] [47].
SERS Enhancement Mechanisms and Substrate Architectures
Fundamental Enhancement Principles
The exceptional sensitivity of SERS stems from the synergistic combination of electromagnetic and chemical enhancement mechanisms. Electromagnetic enhancement (EM), contributing approximately 10â´-10⸠to overall signal amplification, arises from localized surface plasmon resonance (LSPR) effects when incident light interacts with noble metal nanostructures [48] [2]. This interaction generates dramatically enhanced electromagnetic fields at specific nanoscale regions known as "hot spots," typically occurring at nanoparticle tips, edges, and interparticle gaps [47]. Research indicates that although molecules in hot spot regions constitute less than 1% of the total adsorbed molecules, they contribute over 50% of the total SERS signal intensity [48]. The lightning rod effect further concentrates electromagnetic fields at sharp nanostructural features, while propagating surface plasmon polaritons enhance signals along extended metallic surfaces [2].
Chemical enhancement (CM), typically contributing 10¹-10³ to signal amplification, involves charge transfer between the substrate and analyte molecules when adsorbed on the metallic surface [48] [27]. This mechanism alters the polarizability of the analyte molecules, effectively increasing their Raman scattering cross-sections. The chemical enhancement mechanism exhibits molecular specificity, as it depends on the electronic structure of both the substrate and analyte molecules [48]. In practical SERS applications, both electromagnetic and chemical enhancement mechanisms operate concurrently, with electromagnetic effects generally dominating the overall signal amplification [48] [2].
Figure 1: SERS enhancement mechanisms showing electromagnetic (EM) and chemical (CM) pathways
Substrate Architecture Comparison
SERS substrate architectures have evolved significantly from simple colloidal nanoparticles to sophisticated three-dimensional structures engineered to maximize hot spot density and analyte accessibility.
Colloidal substrates typically consist of silver or gold nanoparticles (AgNPs/AuNPs) in suspension, forming reversible aggregates through salt-induced aggregation to create interparticle hot spots [48]. These substrates offer straightforward synthesis and high enhancement factors but suffer from poor reproducibility due to aggregation heterogeneity and sensitivity to environmental conditions [48].
Two-dimensional solid substrates comprise metallic nanostructures immobilized on planar surfaces (e.g., silicon, glass) through lithography, self-assembly, or electrochemical deposition [1]. These systems provide improved stability and better reproducibility compared to colloidal suspensions but offer limited surface area and restricted hot spot distribution confined primarily to the planar surface [1].
Three-dimensional substrates represent the most advanced architecture class, extending nanoplasmonic structures into the z-dimension through vertically aligned nanowires, dendritic frameworks, porous aerogels, or core-shell spheres [1]. These substrates dramatically increase hot spot density volumetrically, enhance analyte capture efficiency through improved diffusion pathways, and enable significantly higher enhancement factors (routinely >10â¸) compared to 2D systems [1]. The porous nature of 3D substrates facilitates analysis of complex biological matrices like blood, saliva, or food extracts by providing interconnected channels for analyte transport while excluding larger interfering components [1].
Table 2: Structural and performance characteristics of SERS substrate architectures
| Architecture | Hot Spot Distribution | Enhancement Factor | Reproducibility (RSD) | Analyte Accessibility | Fabrication Complexity |
|---|---|---|---|---|---|
| Colloidal | Random, aggregation-dependent [48] | 10â¶-10â¹ [48] | Moderate to Poor (>15%) [48] | High for small molecules [48] | Low [48] |
| 2D Solid | Planar, surface-confined [1] | 10âµ-10â· [1] | Moderate (10-15%) [1] | Limited by surface diffusion [1] | Moderate [1] |
| 3D Nanostructured | Volumetric, dense distribution [1] | >10⸠[1] | High (<10%) [1] | Enhanced via porous networks [1] | High [1] |
| Ag-pSi Microarray | Patterned spots with homogeneous signals [50] | 1.75×10ⷠ[50] | High (RSD 8.4%) [50] | Controlled through pore size [50] | High (lithography required) [50] |
Experimental Protocols for SERS-Based Biotoxin Detection
Ag-pSi Microarray Fabrication and Mycotoxin Detection Protocol
The silver-coated porous silicon (Ag-pSi) microarray represents a sophisticated SERS substrate architecture optimized for multiplex mycotoxin detection. The fabrication begins with pre-patterning a silicon wafer using a non-clean room-based lithography process to create a microarray configuration with 25 effective sensing spots per chip [50]. A single-step electrochemical anodization process follows, conducted at 60 mA cmâ»Â² for 12 seconds to produce highly porous nanostructures with controlled pore morphology [50]. The freshly prepared pSi substrates undergo immersion in 1 mM silver nitrate solution (50% ethanol), where the silicon hydride-terminated surface reduces Ag⺠ions to form silver nanoparticles distributed across the porous void [50]. Morphological characterization through high-resolution scanning electron microscopy (HR-SEM) and energy-dispersive X-ray spectroscopy (EDX) confirms optimal silver distribution with high-density coalescent metal island formation [50].
For mycotoxin detection, the Ag-pSi substrate undergoes surface functionalization with specific anti-mycotoxin aptamers through covalent immobilization techniques [50]. The experimental workflow involves: (1) Sample extraction from food matrices (wheat, maize, rice) using appropriate solvents; (2) Incubation of extracts on the functionalized Ag-pSi platform for 15 minutes to facilitate selective biorecognition; (3) Washing to remove unbound components; (4) SERS measurement using a portable Raman instrument with a 785 nm laser excitation source; (5) Spectral analysis targeting characteristic mycotoxin vibrational fingerprints [50]. The method demonstrates low limits of detection (0.922, 0.547, and 0.008 ppb for ochratoxin A, fumonisin B1, and aflatoxin B1, respectively) across a linear range of 0.001-1000 ppb, with recovery values of 95-104% in spiked food samples and RSD below 6.6% [50].
Figure 2: Ag-pSi microarray fabrication and detection workflow
Multimodal SERS Detection Protocol for Marine Biotoxins
Multimodal SERS detection integrates Raman spectroscopy with complementary transduction mechanisms (colorimetry, fluorescence, electrochemistry) to enhance reliability and accuracy in complex marine toxin analysis. A typical protocol for saxitoxin (STX) detection utilizing magnetic SERS nanoprobes involves: (1) Synthesis of Fe₃O₄@Ag core-shell nanoparticles through co-precipitation and silver reduction, creating magnetic plasmonic nanostructures; (2) Functionalization with STX-specific aptamers using thiol chemistry; (3) Sample preparation with shellfish tissue homogenization and rapid extraction; (4) Incubation of samples with functionalized SERS nanoprobes for 30 minutes; (5) Magnetic separation to concentrate toxin-bound complexes while eliminating matrix interferents; (6) Multimodal signal acquisition including SERS spectra, colorimetric readout, and electrochemical measurements [47] [49].
This integrated approach leverages the individual strengths of each detection modality: SERS provides molecular fingerprint identification, colorimetric analysis enables rapid visual screening, and electrochemical detection offers precise quantification [49]. The self-verification capability across multiple signal channels significantly reduces false-positive results common in single-mode biosensors [47]. The method demonstrates detection limits of 0.05 μg/kg for saxitoxin in shellfish matrices, surpassing regulatory requirements with analysis completion within 40 minutes [49].
Advanced SERS Detection Modalities
Label-Free Versus Labeled Detection Strategies
SERS detection methodologies fall into two primary categories: label-free and labeled approaches, each with distinct operational principles and application domains. Label-free detection directly measures the intrinsic Raman spectra of target molecules adsorbed onto SERS-active surfaces, leveraging the natural vibrational fingerprints of the analytes for identification and quantification [51]. This approach works optimally for molecules with relatively large Raman scattering cross-sections that can directly adsorb to metallic surfaces, such as certain pesticides (thiram, thiabendazole) and structural proteins [51]. The primary advantages of label-free detection include simplified assay design, preservation of biomolecular activity, and direct structural information about the target molecule [51].
Labeled SERS detection employs Raman reporter molecules that generate strong, characteristic signals to indirectly quantify target analytes, particularly effective for small molecules with minimal intrinsic Raman scattering, such as most mycotoxins and marine biotoxins [51]. This approach typically incorporates molecular recognition elements (antibodies, aptamers) for specific target capture and quantification. Labeled detection strategies are further categorized into spatial separation detection (distinct capture zones for different analytes) and SERS encoding detection (multiple reporter molecules with non-overlapping spectral signatures) [51]. Common Raman reporter molecules include 4-mercaptobenzoic acid (MBA), 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB), 4-nitrothiophenol (NTP), and 4-aminothiophenol (ATP), selected for their strong affinity to metal surfaces and distinct spectral features [51].
SERS-Based Multimodal Biosensors
The integration of SERS with complementary detection modalities has created powerful multimodal platforms that overcome limitations inherent to single-method approaches. SERS-fluorescence combinations leverage the high sensitivity of fluorescence with the molecular specificity of SERS, utilizing materials like dye-incorporated metal-organic frameworks (MOFs) or quantum dot-plasmonic nanostructures [47] [49]. These systems typically employ fluorescence for rapid screening and SERS for confirmatory analysis, significantly enhancing detection reliability in complex seafood matrices prone to autofluorescence interference [49].
SERS-colorimetric platforms exploit the distinct color changes of gold and silver nanoparticles during aggregation states alongside SERS measurements, providing both visual qualitative assessment and quantitative Raman analysis [47] [49]. This approach is particularly valuable for field testing where instrumental access may be limited, as color changes offer immediate preliminary results while SERS provides definitive identification and precise quantification [49].
SERS-electrochemical systems combine the label-free quantification capabilities of electrochemistry with the structural identification power of SERS, creating synergistic platforms for marine toxin detection [49]. These systems typically employ SERS-active electrodes that function simultaneously as electrochemical sensors and Raman substrates, enabling simultaneous voltammetric and spectroscopic characterization from the same sensing interface [49].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Essential research reagents and materials for SERS-based biotoxin detection
| Reagent/Material | Function | Application Examples | Key Characteristics |
|---|---|---|---|
| Gold Nanoparticles (AuNPs) | Plasmonic core for EM enhancement [48] [47] | Colloidal substrates, labeled probes [48] | Tunable LSPR (500-800 nm), high stability, easy functionalization [48] |
| Silver Nanoparticles (AgNPs) | High enhancement factor substrate [48] [50] | Ag-pSi microarrays, sol-based detection [50] | Strong plasmonic response, higher EF than Au but prone to oxidation [48] |
| Specific Aptamers | Molecular recognition elements [50] | Functionalization of SERS substrates [50] | High affinity/selectivity, thermal stability, reusable after denaturation [50] |
| Raman Reporters (MBA, DTNB) | Signal generation in labeled detection [51] [50] | SERS encoding, multiplex detection [51] | Strong affinity to metals (thiol groups), distinct Raman fingerprints [51] |
| Magnetic Nanoparticles (Fe₃O₄) | Target concentration/separation [47] [49] | Sample pretreatment, matrix interference reduction [49] | Enables magnetic separation, core for core-shell structures [47] |
| Porous Silicon (pSi) | High surface area substrate template [50] | Ag-pSi microarray platforms [50] | Extensive internal surface, straightforward fabrication, versatile modification [50] |
| Cellulose Membranes | Flexible substrate support [27] | Field-deployable sensors, irregular surfaces [27] | Low background signal, biodegradability, mechanical flexibility [27] |
| Metal-Organic Frameworks (MOFs) | Nanoporous concentrator structures [2] [27] | Analyte preconcentration, hybrid substrates [2] | Ultrahigh surface area, molecular sieving, functionalizable pores [2] |
| 3',4',7-Trimethoxyquercetin | 3',4',7-Trimethoxyquercetin, CAS:6068-80-0, MF:C18H16O7, MW:344.3 g/mol | Chemical Reagent | Bench Chemicals |
| ST638 | ST638|Tyrosine Kinase Inhibitor|For Research Use | Bench Chemicals |
The comprehensive evaluation of SERS substrates for marine biotoxin and mycotoxin detection reveals a rapidly advancing field transitioning from laboratory demonstration toward practical implementation. Performance comparisons clearly establish that engineered SERS platforms—particularly 3D nanostructures and functionalized microarrays—deliver sensitivity competitive with gold-standard chromatographic methods while offering superior analysis speed, portability, and multiplexing capabilities. The Ag-pSi microarray exemplifies this progress, achieving detection limits of 0.008 ppb for aflatoxin B1 with excellent reproducibility (RSD <8.4%) and validation in complex food matrices [50].
The emerging paradigm of SERS-based multimodal detection represents the most promising development direction, effectively addressing limitations of individual sensing modalities through integrated signal readouts [47] [49]. These platforms leverage complementary advantages of different spectroscopic techniques to provide self-verifying, highly reliable analyses essential for regulatory decision-making. Future research priorities should focus on developing low-cost, scalable substrate manufacturing methods; enhancing anti-interference capabilities for complex environmental matrices; establishing standardized validation protocols; and integrating artificial intelligence for automated spectral analysis [48] [49]. As these advancements mature, SERS technology is positioned to become the core analytical solution for on-site monitoring of biotoxins, fundamentally transforming environmental safety monitoring and public health protection.
Identification of Microplastics and Persistent Organic Pollutants (POPs)
Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique for the detection of environmental pollutants, offering unparalleled sensitivity and molecular specificity. This guide provides a comparative analysis of SERS substrate performance for identifying two critical pollutant classes: microplastics (MPs) and persistent organic pollutants (POPs). As environmental monitoring faces increasing challenges from these contaminants, SERS technology presents distinct advantages over conventional methods, including minimal sample preparation, potential for field deployment, and extremely low detection limits [2] [52]. This evaluation focuses on substrate design, experimental protocols, and performance metrics to assist researchers in selecting appropriate methodologies for their specific detection needs.
The fundamental principle of SERS relies on the dramatic enhancement of Raman signals when analyte molecules are located near plasmonic nanostructured surfaces. This enhancement stems from two primary mechanisms: electromagnetic enhancement (dominant, resulting from localized surface plasmon resonance) and chemical enhancement (from charge transfer between substrate and analyte) [2] [52]. The electromagnetic enhancement, which can amplify signals by factors of 10^4-10^6, occurs predominantly at "hot spots" - nanoscale gaps between metallic nanostructures where electromagnetic fields are intensely concentrated [53]. Understanding these mechanisms is crucial for optimizing SERS substrates for different pollutant classes.
Comparative Performance of SERS Substrates
The effectiveness of SERS detection varies significantly depending on substrate composition, morphology, and functionalization, as well as the target pollutant class. The following analysis compares representative SERS approaches for MPs and POPs detection, highlighting key performance metrics and applications.
Table 1: Performance Comparison of SERS Substrates for Microplastics Detection
| SERS Substrate | Target Microplastics | Enhancement Factor (EF) | Limit of Detection (LOD) | Real Sample Application |
|---|---|---|---|---|
| Porous Si@Au [54] | Polystyrene | ~10^6 | Single nanoparticle (in 1 mL) | Groundwater |
| AgNPs on regenerated cellulose [52] | Polystyrene | N/R | 0.1 mg/mL | Standard solution |
| Au-functionalized glass slides [52] | Polystyrene, PET | N/R | 10 µg/mL | Standard solution |
| Ag@Au nanostars @ AAO [52] | Polystyrene | N/R | 0.05 mg/g | Tap, river, and seawater |
Table 2: Performance Comparison of SERS Substrates for POPs Detection
| SERS Substrate | Target POPs | Enhancement Factor (EF) | Limit of Detection (LOD) | Real Sample Application |
|---|---|---|---|---|
| D-shaped PM-PCF with Au nanolayer [55] | 2,3,7,8-TCDD (dioxin) | N/R | 1.35 × 10^(-11) M | Standard solution |
| D-shaped PM-PCF with ZrO₂ NPs [55] | 2,3,7,8-TCDD (dioxin) | 3.4 × 10^6 | 8.2 × 10^(-12) M | Standard solution |
| Au@Ag nanocuboids [8] | Organic dyes | N/R | 8.7 × 10^(-10) M | Fishpond water |
| Ag/ZIF-67/TiO₂/Cu [8] | Pesticides (4-ATP) | N/R | 5 × 10^(-11) M | River water |
| Ag-GA [8] | Herbicides (2,4-D) | N/R | 1.5 × 10^(-10) M | Mineral/river water |
N/R = Not Reported
Analysis of Comparative Performance
The tabulated data reveals distinct trends in SERS substrate optimization for different pollutant classes. For microplastics detection, the primary challenge lies in the size discrepancy between MPs (micrometer-scale) and SERS hot spots (nanometer-scale), which limits interaction efficiency [54]. Innovative approaches such as thermal annealing of MPs onto porous substrates have demonstrated remarkable improvements, enabling detection down to single nanoparticles in complex matrices [54].
For POPs detection, the focus shifts toward maximizing enhancement factors and specificity through advanced substrate engineering. Hybrid substrates incorporating metals with functional materials like MOFs (Metal-Organic Frameworks) or graphene show particular promise, offering both electromagnetic enhancement and additional chemical enhancement through charge transfer mechanisms [8] [2]. The integration of optical fiber platforms further enhances detection capabilities by improving light management and enabling field analysis [55].
Cellulose-based substrates represent an emerging category with significant potential for both application areas, offering advantages including flexibility, biodegradability, low background signal, and cost-effectiveness [27]. These substrates can be functionalized with metal nanoparticles to create flexible SERS platforms adaptable to irregular surfaces, significantly improving sample contact and signal collection efficiency [27] [53].
Experimental Protocols and Methodologies
SERS Substrate Preparation Protocols
Plasmonic Porous Silicon Substrates for MPs Detection [54]:
- Begin with p-type silicon (100) wafer with low resistivity
- Clean wafer thoroughly and electrochemically etch in HF:DMF solution (1:20 ratio)
- Apply etching parameters: 2 mA current for 15 minutes
- Rinse etched wafer with ethanol and dry completely
- Deposit Au layer using DC magnetron sputtering (Ar plasma, 4 Pa pressure, 7.5 W discharge power, 300 s sputtering time, 40 mA current)
- Characterize enhancement factor (approximately 10^6) using standard analytes
D-shaped Polarization-Maintaining Photonic Crystal Fiber (PM-PCF) for POPs [55]:
- Cut 7 cm segment of commercial PM-PCF (hole diameter: 2.2-4.4 μm, pitch: 4.4 μm)
- Strip fiber coating to create 1 cm sensing length
- Polish sensing length manually using polishing sheets with decreasing grit sizes (12 μm, 9 μm, 3 μm)
- Monitor polishing depth using optical microscopy
- Deposit 40 nm Au nanolayer using magnetron sputtering system
- For dual-substrate enhancement, mix ZrOâ‚‚ NPs (20 nm) with analyte solution
Cellulose-Based Substrate Fabrication [27]:
- Select appropriate cellulose form (nanofibers, crystals, or bacterial cellulose)
- Functionalize surface with metal nanoparticles (Ag, Au) through various deposition techniques
- Control nanoparticle density and distribution to optimize hot spot formation
- Ensure homogeneous plasmonic surface through appropriate pre-treatment methods
Sample Processing and Measurement Protocols
Microplastics Analysis with Thermal Treatment [54]:
- Deposit 10 μL of MP solution (10^4 to 10^8 particles/L) onto SERS substrate
- Apply thermal treatment (50-150°C) to induce polymer penetration into substrate pores
- Cool samples to room temperature before SERS measurements
- For real environmental samples, filter water through 10 nm filter to remove natural nanoparticles
- Spike with known MP concentrations for quantification studies
- Acquire multiple spectra from different substrate regions
POPs Detection in Aqueous Matrices [8] [55]:
- For organic pollutants (dyes, pesticides, herbicides): Directly deposit sample solution onto substrate
- For dioxin compounds: Employ concentration steps or pre-treatment if necessary
- Incubate to allow adequate adsorption onto substrate surface
- Rinse gently to remove non-specifically bound compounds
- Conduct SERS measurements using appropriate laser wavelengths (532-785 nm)
- Utilize mapping approaches for improved statistical reliability
Signaling Pathways and Experimental Workflows
The detection mechanisms for MPs and POPs involve distinct pathways and workflows, as illustrated in the following diagrams:
Research Reagent Solutions and Essential Materials
Successful implementation of SERS-based detection requires specific materials and reagents optimized for different pollutant classes. The following table details essential components for developing effective SERS methodologies:
Table 3: Essential Research Reagents for SERS-Based Pollutant Detection
| Reagent Category | Specific Examples | Function in SERS Analysis |
|---|---|---|
| Plasmonic Materials | Gold, silver nanoparticles | Generate electromagnetic enhancement through LSPR |
| Support Substrates | Porous silicon, cellulose, anodized aluminum oxide | Provide structural foundation for plasmonic materials |
| Functional Materials | ZIF-67, TiOâ‚‚, graphene oxide, MOFs | Enhance adsorption, provide chemical enhancement |
| Target Pollutants (MPs) | Polystyrene, polyethylene, polypropylene, PET | Model compounds for method development |
| Target Pollutants (POPs) | 2,3,7,8-TCDD, nitrobenzene, pesticides, herbicides | Representative persistent organic pollutants |
| Surface Modifiers | Molecularly imprinted polymers, aptamers, thiols | Improve selectivity and binding affinity |
| Reference Compounds | Rhodamine 6G, 4-aminothiophenol | Substrate performance calibration |
SERS technology represents a transformative approach for detecting microplastics and persistent organic pollutants in environmental matrices. The comparative analysis presented in this guide demonstrates that substrate selection must be tailored to the specific pollutant class, with thermal-assisted approaches showing particular promise for MPs and hybrid substrates excelling for POPs detection. Current challenges include substrate reproducibility, matrix interference in complex environmental samples, and the need for standardized validation protocols [53] [2].
Future directions point toward increased integration of artificial intelligence for spectral analysis [54] [56], development of multi-functional composite substrates [2], and creation of portable, field-deployable systems for real-time environmental monitoring [53] [56]. As SERS technology continues to mature, it holds significant potential to become a mainstream analytical technique for environmental protection agencies, research institutions, and regulatory bodies tasked with monitoring these pervasive pollutants.
Surface-Enhanced Raman Spectroscopy (SERS) has transitioned from a research tool to a powerful analytical technique for environmental pollutant detection, driven primarily by innovations in substrate technology. The development of flexible, stretchable, and reusable SERS substrates represents a critical platform innovation, enabling practical field applications that traditional rigid substrates cannot support. These advanced substrates combine the exceptional sensitivity of SERS—capable of single-molecule detection—with the mechanical properties required for real-world environmental monitoring, such as conformal contact with irregular surfaces, durability, and cost-effectiveness through multiple uses [57] [2] [27].
For researchers and drug development professionals working on environmental pollutant detection, the evolution of these substrates addresses longstanding challenges in reproducibility, matrix interference, and on-site deployment. This guide objectively compares the performance of emerging flexible SERS platforms against conventional alternatives, providing critical experimental data and protocols to inform substrate selection for specific application requirements.
Performance Comparison of SERS Substrate Platforms
The performance characteristics of SERS substrates vary significantly across different platform types. The following tables provide a comprehensive comparison of traditional and emerging substrate technologies for environmental sensing applications.
Table 1: Comprehensive Comparison of SERS Substrate Types for Environmental Detection
| Feature | Traditional 2D Rigid Substrates | 3D Nanostructured Substrates | Flexible/Stretchable Substrates | Reusable Substrates |
|---|---|---|---|---|
| Enhancement Factor | 10$^5$-10$^7$ [1] | >10$^8$ [1] | 10$^7$-10$^11$ [27] | Varies with regeneration method |
| Reproducibility (RSD) | Moderate [1] | High (<10%) [1] | 5-15% [57] [27] | Dependent on cycle count |
| Analyte Accessibility | Limited surface diffusion [1] | Enhanced 3D diffusion [1] | Conformal contact [27] | May decrease with reuse |
| Mechanical Properties | Rigid, brittle | Variable | Flexible, stretchable [27] | Varies with platform |
| Fabrication Complexity | Moderate [58] | High [58] [1] | Low to moderate [27] | Often complex |
| Cost Effectiveness | Single-use | Single-use | Potential reuse | Designed for multiple uses |
| Field Deployment | Limited | Limited | Excellent [27] | Good with regeneration |
Table 2: Quantitative Performance Data for Specific SERS Substrates
| Substrate Type | Detection Limit | Enhancement Factor | Reproducibility (RSD) | Key Analytes Demonstrated |
|---|---|---|---|---|
| Photonic Crystal Substrates [57] | 30 ppb (cocaine) | 10$^4$-10$^6$ [57] | <5% [57] | Illicit drugs, pharmaceuticals |
| Cellulose-based Flexible [27] | Single molecule [27] | Up to 10$^11$ [27] | Not specified | Pesticides, contaminants |
| Hydrogel-based 3D [1] | 0.838 pM (UO$_2^{2+}$) [1] | >10$^8$ [1] | <10% [1] | Heavy metals, glucose |
| Metal Nanowire Arrays [58] | Not specified | High (nanowire-dependent) | 3% across substrate [57] | Biomolecules, dyes |
| Star-shaped Nanoparticles [58] | Not specified | Tunable via spike geometry | High batch reproducibility [58] | Biomedical applications |
Table 3: Comparison of Flexibility and Reusability Performance Metrics
| Substrate Type | Bending Cycles | Signal Retention | Reuse Cycles | Cleaning Method |
|---|---|---|---|---|
| Cellulose-based [27] | >100 | >90% | Limited | Solvent rinsing |
| Hydrogel-based [1] | Not specified | Responsive to stimuli | 5-10 | Buffer exchange |
| Polymer-supported Metal NPs | 50-200 | 80-95% | 3-8 | Mild etching |
| 3D Aerogels [1] | Brittle | High | 10+ | Thermal treatment |
Experimental Protocols for SERS Substrate Evaluation
Standardized experimental protocols are essential for objectively comparing SERS substrate performance. The following methodologies represent current best practices for evaluating flexible, stretchable, and reusable substrates for environmental applications.
Substrate Fabrication and Functionalization
Cellulose-Based Flexible Substrates [27]:
- Protocol: Cellulose nanocrystals are extracted from plant material or agricultural waste via acid hydrolysis. The resulting nanocellulose suspension is vacuum-filtered or cast to form flexible films. Functionalization is achieved by immersing films in noble metal nanoparticle (Au, Ag) suspensions under controlled agitation. Surface modification with silane coupling agents can enhance nanoparticle adhesion.
- Key Parameters: Nanoparticle density (10-100 particles/μm²), nanogap control (<10 nm for hot spots), and uniform distribution across cellulose fibers are critical for performance.
- Quality Control: SEM imaging to verify nanoparticle distribution, Raman mapping to assess signal uniformity, and mechanical testing to confirm flexibility.
Hydrogel-Based 3D SERS Substrates [1]:
- Protocol: Prepolymer solution containing acrylamide monomers, crosslinker, and photoinitiator is mixed with pre-synthesized Au or Ag nanoparticles. The mixture is poured into molds and UV-polymerized. For stimulus-responsive substrates, functional monomers (e.g., acrylic acid) are incorporated.
- Key Parameters: Crosslinking density (affects pore size and analyte diffusion), nanoparticle concentration (0.1-1 nM), and swelling ratio in different environments.
- Quality Control: Swelling tests in various pH buffers, compression testing, and SERS signal stability over time.
Analytical Performance Assessment
Enhancement Factor Calculation [27]:
- Protocol: EF = (ISERS / NSERS) / (IRaman / NRaman), where ISERS and IRaman are the SERS and normal Raman intensities of a specific vibrational mode, and NSERS and NRaman are the number of molecules probed under SERS and normal conditions.
- Standard Analytes: Rhodamine 6G (10$^{-6}$ to 10$^{-9}$ M) or pyridine (1% to 1 mM) are commonly used reference compounds.
- Experimental Setup: Identical instrument parameters (laser power, integration time, objective) must be used for both SERS and normal Raman measurements.
Reproducibility Assessment [57]:
- Protocol: Collect SERS spectra from at least 20 random locations on the substrate using the same analyte and concentration. Calculate the relative standard deviation (RSD) of characteristic peak intensities.
- Acceptance Criteria: For analytical applications, RSD should be <10-15% [57] [1]. High-performance substrates demonstrate RSD <5% [57].
Reusability Testing:
- Protocol: After initial SERS measurement, substrates are regenerated using appropriate cleaning methods (solvent rinsing, plasma treatment, or chemical etching). The SERS signal intensity of a standard analyte is measured after each regeneration cycle.
- Performance Metric: Number of cycles until signal intensity drops to 80% of initial value.
Environmental Application Testing
Detection of Heavy Metals [1] [2]:
- Protocol: Functionalize SERS substrates with chelating agents (e.g., EDTA derivatives) or DNAzymes specific to target heavy metals (Pb$^{2+}$, Hg$^{2+}$, UO$_2^{2+}$). Incubate in contaminated water samples, then measure SERS signal changes.
- Detection Limits: Compare achieved detection limits against regulatory thresholds (e.g., EPA drinking water standards).
- Protocol: Deploy flexible SERS substrates directly on fruit/vegetable surfaces or in water samples. For complex matrices, incorporate separation layers to reduce interference.
- Validation: Compare results with LC-MS/MS reference methods.
Signaling Pathways and Enhancement Mechanisms
The exceptional performance of advanced SERS substrates stems from complex interplay between electromagnetic and chemical enhancement mechanisms, which are further optimized in flexible and 3D architectures.
Electromagnetic Enhancement Mechanism
The electromagnetic (EM) enhancement mechanism dominates SERS effects, contributing up to 10$^6$-10$^8$ signal amplification [2]. When incident photons interact with metallic nanostructures, they excite localized surface plasmon resonance (LSPR)—collective oscillations of conduction electrons at the metal-dielectric interface [58] [2]. In flexible substrates with controlled nanogaps (1-10 nm), these oscillations generate intensely localized electromagnetic fields known as "hot spots" [27]. The "lightning rod effect" further enhances fields at sharp nanostructure features, explaining the high performance of star-shaped nanoparticles and nanocubes [58] [2].
Three-dimensional SERS substrates significantly outperform 2D platforms by creating a volumetric distribution of hot spots throughout their structure rather than confining them to a single plane [1]. This architecture increases the probability of analyte molecules encountering enhancement regions, thereby boosting sensitivity and reproducibility. The flexibility of substrates like cellulose and hydrogels enables optimal orientation of hot spots toward analytes on irregular surfaces, a critical advantage for field applications [27].
Chemical Enhancement Mechanism
Chemical enhancement provides more modest signal amplification (10-10$^3$) but contributes significantly to molecular specificity [2] [27]. This mechanism involves charge transfer between analyte molecules and the metal substrate when molecules adsorb to the surface. The formation of chemical bonds creates new electronic states that resonate with both incident laser energy and molecular vibrational energies, effectively increasing the Raman scattering cross-section.
In functionalized flexible substrates, chemical enhancement can be optimized by incorporating materials with high analyte affinity—such as metal-organic frameworks (MOFs) on cellulose—that preconcentrate target molecules near enhancement regions [27]. This synergistic approach is particularly valuable for detecting environmental pollutants at trace concentrations in complex matrices.
Research Reagent Solutions for SERS Substrate Development
The development and application of advanced SERS substrates requires specialized materials and reagents. The following table catalogs essential solutions for researchers working in this field.
Table 4: Essential Research Reagents for Flexible SERS Substrate Development
| Reagent/Material | Function | Example Applications | Key Characteristics |
|---|---|---|---|
| Noble Metal Nanoparticles [58] [27] | Plasmonic enhancement | SERS active layer | Au (high stability), Ag (high enhancement), tunable LSPR |
| Cellulose Nanocrystals [27] | Flexible substrate matrix | Sustainable SERS platforms | Biodegradable, low background, mechanical flexibility |
| Silane Coupling Agents [58] [27] | Surface functionalization | NP attachment to substrates | Improve adhesion, control NP density |
| Hydrogel Polymers [1] | 3D flexible matrix | Stimuli-responsive substrates | Tunable porosity, swelling behavior |
| Metal-Organic Frameworks [27] | Molecular recognition | Selective analyte capture | High surface area, specific affinity |
| Rhodamine 6G [27] | SERS calibration | Substrate performance testing | Standard reference, well-characterized spectrum |
| Ascorbic Acid [58] | Reducing/capping agent | NP synthesis | Shape-controlled synthesis (e.g., nanostars) |
The ongoing innovation in flexible, stretchable, and reusable SERS substrates addresses critical limitations of traditional platforms for environmental pollutant detection. Cellulose-based materials offer an exceptional balance of sustainability, flexibility, and performance [27], while hydrogel-based substrates enable 3D hot spot distribution and stimulus-responsive detection [1]. Photonic crystal designs provide unparalleled reproducibility through semiconductor manufacturing techniques [57].
For researchers selecting SERS platforms, the optimal choice depends on specific application requirements: cellulose-based substrates for sustainable, flexible monitoring; hydrogel platforms for dynamic sensing in aqueous environments; and reusable designs for cost-effective, repeated measurements. As fabrication techniques advance, these innovative substrates will continue to bridge the gap between laboratory demonstration and practical field deployment, ultimately enhancing our capability to monitor and address environmental pollution with unprecedented sensitivity and specificity.
Overcoming Analytical Challenges: Strategies for Enhanced SERS Performance and Reproducibility
Addressing Substrate Reproducibility and Batch-to-Batch Variability
Surface-Enhanced Raman Scattering (SERS) has emerged as a powerful analytical technique for detecting environmental pollutants, offering single-molecule sensitivity and molecular fingerprinting capabilities [59] [2]. However, its transition from laboratory curiosity to routine analytical tool is severely hampered by substrate reproducibility issues and significant batch-to-batch variability [59] [60]. These challenges stem primarily from the heterogeneous distribution of electromagnetic "hot spots"—nanoscale regions where plasmonic fields are intensely concentrated—across SERS substrates [59]. For environmental pollutant detection, where reliable quantification of pesticides, pharmaceuticals, and heavy metals is crucial, this variability poses a fundamental limitation [8]. This guide objectively compares SERS substrate strategies based on their effectiveness in overcoming reproducibility challenges, providing researchers with experimental data and methodologies to inform substrate selection for environmental sensing applications.
Fundamental Mechanisms Underlying SERS Variability
The reproducibility problem in SERS originates from the fundamental enhancement mechanisms and nanofabrication challenges.
Electromagnetic Enhancement Mechanism: This dominant mechanism provides enhancement factors of 10³–10⸠through localized surface plasmon resonance (LSPR) occurring when incident light matches the oscillation frequency of conduction electrons in noble metals [61] [2]. The random distribution and density of "hot spots" – nanogaps between nanoparticles, sharp tips, or irregular structures – cause dramatic signal variations, as the hottest SERS-active sites are sparsely distributed yet contribute disproportionately to overall intensity [60].
Chemical Enhancement Mechanism: This secondary mechanism provides more modest enhancement (up to ~10³) through charge transfer between analyte molecules and the substrate surface when molecules are chemically adsorbed [61] [2]. While potentially more uniform, chemical enhancement depends strongly on the chemical affinity between specific pollutants and substrate materials, creating variability across different environmental analytes [61].
Table 1: Sources of Reproducibility Challenges in SERS Substrates
| Variability Source | Impact on Reproducibility | Affected Performance Metrics |
|---|---|---|
| Hot Spot Distribution | Random spatial arrangement of high-enhancement zones | Point-to-point signal variance (10-60% CV typically) |
| Nanoparticle Synthesis | Batch-to-batch differences in size, shape, and aggregation | Enhancement factor fluctuations between substrate batches |
| Surface Contamination | Non-specific binding and fouling in complex environmental matrices | Signal drift and reduced analyte affinity |
| Fabrication Inconsistency | Difficulties in replicating identical nanostructures at scale | Inter-substrate and inter-batch performance differences |
Comparative Analysis of SERS Substrate Strategies
Various substrate strategies have been developed to address reproducibility challenges, each with distinct advantages and limitations for environmental pollutant detection.
Traditional Noble Metal Substrates
Traditional substrates based on gold (Au), silver (Ag), and copper (Cu) nanoparticles or nanostructures remain widely used due to their strong plasmonic properties in the visible spectrum [61] [62].
Performance Characteristics: Silver typically provides the highest enhancement factors but suffers from oxidation and sulfidation in environmental samples [62]. Gold offers better stability but at higher cost [62]. These substrates consistently achieve detection limits of 10â»â¹ to 10â»Â¹Â² M for organic dyes like malachite green and crystal violet in water samples [8].
Reproducibility Limitations: The predominant challenge with traditional substrates is the uncontrolled, random distribution of hot spots. As noted in research, "the hottest SERS-active sites account for only 63 in every 1,000,000 sites, but contribute 24% of the overall SERS intensity" [60]. This extreme heterogeneity results in coefficients of variation (CV) typically ranging from 10% to 60% between measurement spots and across different substrate batches [59].
Flexible SERS Substrates
Flexible SERS substrates (FSS) represent an emerging class of materials that offer enhanced versatility for environmental sampling on irregular surfaces [61].
Material Composition: FSS incorporate plasmonic nanostructures onto deformable supports including polymers (PDMS, PET), paper-based materials, textiles, and biomaterials [61]. These substrates enable conformal contact with rough environmental surfaces like fruit skins (for pesticide detection) or filtration membranes [61].
Reproducibility Profile: While FSS excel in adaptability and cost-effective large-area fabrication, they face challenges in controlling nanogap uniformity across flexible matrices. Their primary advantage lies in sample collection efficiency rather than intrinsic signal reproducibility, though some studies report improved consistency through periodic nanostructures transferred to flexible supports [61].
Table 2: Performance Comparison of SERS Substrate Types for Environmental Pollutant Detection
| Substrate Type | Enhancement Factor Range | Typical LOD for Pollutants | Reproducibility (CV) | Key Environmental Applications |
|---|---|---|---|---|
| Ag-Based Substrates | 10â¶â€“10⸠| 10â»â¹â€“10â»Â¹Â² M (dyes, pesticides) | 10–60% | Organic pollutant detection in wastewater [8] |
| Au-Based Substrates | 10âµâ€“10â· | 10â»â¸â€“10â»Â¹â° M (pesticides, pharmaceuticals) | 15–50% | Heavy metal detection, biosensing [62] |
| Flexible Substrates | 10â´â€“10ⶠ| 10â»â¶â€“10â»â¹ M (pesticides on surfaces) | 20–70% | Swab sampling, wearable environmental sensors [61] |
| Semiconductor Substrates | 10³–10âµ | 10â»â¶â€“10â»â¸ M (dyes, organics) | 5–15% | Stable detection in corrosive environments [60] |
| Hybrid Substrates | 10âµâ€“10â· | 10â»â¸â€“10â»Â¹Â¹ M (various pollutants) | 8–20% | Multiplexed pollutant detection in complex matrices [2] |
Semiconductor and Alternative Substrates
Non-traditional substrates based on semiconductors (TiO₂, WO₃, NiO) and other materials (Al, Si) offer distinct advantages for reproducibility despite typically lower enhancement factors [62] [60].
Reproducibility Advantages: Semiconductor substrates rely primarily on charge-transfer enhancement mechanisms that generate more uniform signal enhancement across the substrate surface [60]. Electrochromic semiconductors like tungsten oxide demonstrate particularly promising reproducibility through their colorimetric functionality, where "a clear quantitative relationship can be found between the SERS enhancement of the colored substrate and the amount of intercalated charges" [60]. This enables visual assessment of SERS activity and controlled enhancement modulation.
Performance Trade-offs: While enhancement factors for semiconductor substrates (10³–10âµ) are generally lower than for noble metals, their reproducibility advantages (5–15% CV) make them valuable for quantitative environmental analysis [60]. Additionally, they offer renewability—electrochromic substrates can be reversibly colored and bleached over 50 cycles with minimal performance degradation [60].
Internal Standardization Strategies
Rather than modifying substrate architecture, internal standardization addresses variability through signal correction methods.
Molecular Internal Standards: Adding reference compounds to samples allows signal normalization but introduces complexity. Analyte-like internal standards may compete for binding sites, while isotope-edited standards (IEIS) are ideal but often unavailable for environmental pollutants [59].
Hot Spot Normalization: This innovative approach uses the surface-enhanced elastic scattering signal from amplified spontaneous emission as an intrinsic internal standard [59] [63]. Both elastic and inelastic scattering undergo identical enhancement at hot spots, enabling direct normalization. This method reduces coefficients of variation from 10–60% down to 2–7% without requiring additional chemicals [59].
Advanced Experimental Protocols for Reproducibility Assessment
Hot Spot Normalization Protocol
Objective: To minimize point-to-point and batch-to-batch variability using intrinsic substrate properties.
Materials:
- SERS substrates (any plasmonic material)
- Raman spectrometer with 785 nm excitation laser
- Long-pass edge filter (cutoff at ~68 cmâ»Â¹)
- Environmental pollutant samples (e.g., chloroanilines, pesticides)
Methodology:
- Acquire SERS spectra using a confocal Raman system in backscattering mode with 10× objective (NA = 0.4)
- Collect spectral maps (e.g., 20 × 20 spectra across 100 × 100 μm² area) with 0.5 s integration time
- Simultaneously measure the low-wavenumber pseudoband (νe) arising from surface-enhanced elastic scattering between 106–146 cmâ»Â¹ [59]
- Process data using MATLAB or similar platform:
- Perform baseline correction on all spectra
- Integrate analyte Raman band intensity (Iáµ£)
- Integrate elastic scattering pseudoband intensity (Iâ‚‘)
- Calculate normalized intensity: Iâ‚™ = Iáµ£/Iâ‚‘
- Compare normalized versus non-normalized coefficient of variation across mapping points
Validation: This protocol demonstrated 80-90% reduction in CV for chloroaniline detection on AuNP/BC substrates, enabling reliable quantification under both static and dynamic conditions [59].
Multivariate Analysis for Enhanced Reprodubility
Objective: To improve reproducibility in complex environmental matrices using full-spectrum chemometric analysis.
Materials:
- SERS mapping capability (2D sample scanning)
- Methotrexate or target pollutant samples
- Commercial serum or environmental matrix
Methodology:
- Collect comprehensive SERS maps rather than single-point spectra
- Apply baseline correction (Standard Normal Variable transformation or Savitzky-Golay smoothing)
- Perform image threshold segmentation to select relevant pixels
- Utilize genetic algorithm wavelength screening to identify analyte-specific spectral regions
- Construct quantitative models using Partial Least Squares Regression
- Validate with spiked environmental samples
Performance: This approach demonstrated 25% higher reproducibility (average RSD 15.6%), 30% improved sensitivity (LOD 5.7 μM), and 110% better accuracy compared to univariate analysis [64].
Experimental Workflow for SERS Reproducibility Assessment
Research Reagent Solutions for Reproducible SERS Sensing
Table 3: Essential Materials and Reagents for Reproducible SERS Substrate Fabrication
| Material/Reagent | Function | Application Examples | Key Considerations |
|---|---|---|---|
| Chloroauric Acid (HAuClâ‚„) | Gold nanoparticle precursor | AuNP synthesis for stable SERS substrates | High purity reduces batch variability; citrate reduction method most common [59] |
| Silver Nitrate (AgNO₃) | Silver nanoparticle precursor | High-enhancement Ag substrates | Prone to oxidation; requires antioxidant coatings for environmental applications [59] [62] |
| Sodium Citrate | Reducing and stabilizing agent | Colloidal nanoparticle synthesis | Concentration determines final nanoparticle size and size distribution [59] |
| Tungsten Oxide Sputtering Targets | Semiconductor substrate fabrication | Electrochromic SERS substrates with high reproducibility | Enables large-scale, uniform film deposition with controlled composition [60] |
| 4-Mercaptobenzoic Acid (4-MBA) | Model analyte and surface functionalization | Substrate performance validation and chemical enhancement studies | Provides consistent Raman signature for enhancement factor calculations [59] |
| Rhodamine 6G | Fluorescent dye and SERS probe | Substrate calibration and performance comparison | Enables cross-laboratory benchmarking of substrate enhancement [60] |
| Aluminum Chloride (AlCl₃) | Electrolyte for cation intercalation | Activation of electrochromic semiconductor substrates | Enables controlled, reversible substrate tuning for renewable sensing [60] |
Addressing substrate reproducibility and batch-to-batch variability remains a critical frontier in SERS research, particularly for environmental pollutant detection where reliable quantification is essential. Current evidence suggests that no single substrate strategy universally outperforms others across all metrics. Traditional noble metals offer superior enhancement but suffer from significant variability, while semiconductor substrates provide excellent reproducibility with more modest sensitivity. The most promising approaches include hybrid strategies that combine materials to leverage both electromagnetic and chemical enhancement mechanisms, alongside advanced normalization and data processing techniques that mathematically compensate for physical substrate inconsistencies.
Future research directions should focus on developing standardized fabrication protocols, establishing universal calibration methodologies, and creating reference materials for cross-platform comparison. Integration of artificial intelligence for substrate characterization and signal processing shows particular promise for overcoming remaining reproducibility challenges. As these advancements mature, SERS technology will transition more fully from laboratory demonstration to routine environmental monitoring, enabling precise, reliable detection of pollutants at trace levels in complex environmental matrices.
Mitigating Matrix Interference in Complex Environmental Samples
Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique for detecting trace environmental pollutants, offering molecular specificity, high sensitivity, and minimal sample preparation requirements [65] [35]. However, its application to complex environmental samples faces a significant challenge: matrix interference, where non-target components compete with analytes for binding sites on SERS substrates, thereby reducing sensitivity and accuracy [66] [67]. This comparison guide objectively evaluates three advanced substrate engineering strategies designed to mitigate these interference effects, providing researchers with experimental data and protocols to inform their selection of appropriate methodologies for environmental pollutant detection.
The core enhancement mechanisms of SERS arise from both electromagnetic and chemical effects. Electromagnetic enhancement, the primary contributor, originates from localized surface plasmon resonance (LSPR) in noble metal nanostructures, creating intense localized electric fields known as "hot spots" [12] [35]. Chemical enhancement involves charge transfer between analyte molecules and the substrate surface, further amplifying the Raman signal [65] [27]. Matrix interference disrupts both mechanisms by preventing target molecules from reaching these active sites, particularly problematic in complex matrices like soil extracts, agricultural runoff, and food samples [66] [35].
Comparative Analysis of SERS Substrate Strategies
The table below compares three strategic approaches for mitigating matrix interference, highlighting their key features and performance metrics.
Table 1: Comparison of SERS Substrate Strategies for Mitigating Matrix Interference
| Strategy | Key Features | Enhancement Mechanism | Reported LOD Improvement | Best For |
|---|---|---|---|---|
| Biorecognition-Enhanced SERS [65] | Integration with antibodies, aptamers, or enzymes; high specificity. | Selective capture of target analytes via biorecognition. | Not specified, but enables detection in complex matrices. | Target-specific applications; complex biological matrices. |
| Paper Centrifugation SERS (PC-SERS) [66] | Uses a rotating paper disk coated with AgNPs for separation and detection. | Differential migration and adsorption during centrifugation. | 100x lower LOD vs. static SERS (for sulfonamide). | Rapid, on-site monitoring of small molecules in liquids. |
| Magnetic Nanoparticle Enrichment [65] [67] | Functionalized magnetic particles (e.g., Fe$3$O$4$) for pre-concentration. | Physical separation and pre-concentration of analytes before SERS detection. | 10$^4$–10$^15$ fold sensitivity increase via enrichment [67]. | Processing large-volume samples with diverse interferents. |
Detailed Experimental Protocols
Protocol: Paper Centrifugation SERS (PC-SERS)
This protocol effectively mitigates competitive adsorption by leveraging differential migration forces [66].
- Step 1: Substrate Preparation. Fabricate a circular paper disk (e.g., chromatography grade) and coat it with silver nanoparticles (AgNPs) to create a P-AgNPs disk, serving as both separation medium and SERS substrate.
- Step 2: Sample Loading and Centrifugation. Apply the liquid sample (e.g., regenerated water or vegetable extract) to the center of the stationary P-AgNPs disk. Initiate rotation at a defined centrifugal force. Solvent molecules and interferents with weaker interaction forces with the P-AgNPs migrate radially outward more quickly than the target analytes.
- Step 3: Zone Formation and Analysis. After a short rotation period (e.g., 20 minutes), distinct zones form on the substrate: a central ring enriched with the target analyte. Stop the centrifugation and perform SERS measurements directly on this analyte-enriched zone.
This method enhances signal intensity by 14-fold within 20 minutes compared to conventional static SERS analysis and achieves a detection limit of 1 μg·Lâ»Â¹ for sulfonamide antibiotics even in the presence of strong interferents [66].
Protocol: Biorecognition-Based SERS Biosensors
This protocol uses molecular recognition for high specificity in complex matrices [65].
- Step 1: Substrate Functionalization. Immobilize biorecognition elements (e.g., antibodies or aptamers specific to a target pesticide like glyphosate or atrazine) onto a plasmonic nanostructure (e.g., gold or silver nanoparticles).
- Step 2: Sample Incubation. Expose the functionalized SERS substrate to the processed environmental sample (e.g., a soil or food extract). Allow time for the target analyte to bind specifically to the capture agents.
- Step 3: Washing and Signal Detection. Rinse the substrate to remove unbound matrix components and interferents. The SERS signal is generated either directly from the captured analyte or indirectly via a Raman reporter molecule linked to the detection complex.
This approach significantly improves selectivity by ensuring that only the target molecule is captured and detected, effectively isolating it from the complex sample matrix [65].
Protocol: SERS with Magnetic Pre-concentration
This protocol uses magnetic nanoparticles for efficient analyte enrichment [65] [67].
- Step 1: Nanoparticle Incubation. Disperse magnetic nanoparticles (e.g., Fe₃O₄), often coated with a functional layer like graphene oxide to enhance π-π interactions with analytes, into a large volume of the environmental sample.
- Step 2: Magnetic Separation. After a sufficient incubation period for analytes to adsorb onto the nanoparticles, apply an external magnetic field to concentrate the nanoparticle-analyte complexes at the bottom of the vessel. Discard the supernatant containing the bulk of the matrix interferents.
- Step 3: SERS Analysis. Re-disperse the concentrated pellet in a small volume of solvent and deposit it onto a standard SERS substrate or analyze it directly if the nanoparticles are plasmonically active.
This enrichment strategy can improve sensitivity by a factor of 10ⴠto 10¹ⵠwhen combined with optimized hotspots, making it exceptionally powerful for trace-level detection [67].
Experimental Workflow and Signaling Pathways
The following diagram illustrates the logical workflow for selecting and applying the discussed SERS strategies to mitigate matrix interference.
The Scientist's Toolkit: Key Research Reagent Solutions
The table below lists essential materials and their functions for implementing the discussed SERS strategies.
Table 2: Key Research Reagents for SERS Interference Mitigation
| Reagent / Material | Function in Experiment | Key Characteristics |
|---|---|---|
| Gold Nanoparticles (AuNPs) [65] [12] | Plasmonic substrate core for signal enhancement. | Biocompatible, tunable LSPR in visible-NIR range, easily functionalized. |
| Silver Nanoparticles (AgNPs) [65] [66] [12] | Plasmonic substrate offering high enhancement factors. | Strong plasmonic activity, cost-effective. |
| Functionalized Magnetic Nanoparticles [65] [67] | Solid-phase extraction and pre-concentration of analytes. | High surface area, superparamagnetic, often coated with polymers or carbon. |
| Cellulose/Paper Substrates [66] [27] | Porous, flexible support for nanoparticles in PC-SERS and other platforms. | Low cost, biodegradable, minimal background Raman signal. |
| Graphene Oxide [65] | Coating material to enhance analyte adsorption via π-π interactions. | Large specific surface area, promotes chemical enhancement (CM). |
| Specific Antibodies/ Aptamers [65] | Biorecognition elements for target-specific capture. | High binding affinity and specificity for a given pesticide or analyte. |
| Tetrapotassium hexacyanoferrate | Tetrapotassium hexacyanoferrate, MF:C6FeK4N6, MW:368.34 g/mol | Chemical Reagent |
| DL-Methylephedrine saccharinate | DL-Methylephedrine Saccharinate|High-Quality Research Chemical | DL-Methylephedrine saccharinate is a sympathomimetic agent for respiratory and neuropharmacology research. For Research Use Only. Not for human or veterinary use. |
The fight against matrix interference in complex environmental samples requires sophisticated substrate engineering strategies. Biorecognition-enhanced SERS offers unparalleled specificity, PC-SERS provides a rapid and effective physical separation mechanism, and magnetic enrichment delivers exceptional sensitivity gains for trace analysis. The optimal choice depends on the specific analytical challenge, including the nature of the matrix, the required detection limit, and available laboratory resources. Future developments will likely focus on integrating these strategies into portable, automated systems and leveraging machine learning for improved spectral interpretation, further solidifying SERS as a robust tool for environmental monitoring [56] [35].
Optimizing Signal-to-Noise Ratio and Minimizing Fluorescence Background
Surface-Enhanced Raman Scattering (SERS) has emerged as a powerful analytical technique for the detection of trace environmental pollutants, offering molecular fingerprinting capabilities and exceptional sensitivity. However, the practical application of SERS in complex environmental matrices is often challenged by high background signals and fluorescence interference, which can obscure the characteristic Raman peaks of target analytes. The signal-to-noise ratio (SNR) and fluorescence background are critically dependent on the properties of the SERS substrate and the experimental methodology. This guide provides a systematic comparison of SERS substrate technologies, focusing on their performance in optimizing SNR and minimizing fluorescence for environmental pollutant detection, to inform researchers and development professionals in their selection process.
Substrate Comparison and Performance Data
The following table summarizes key SERS substrate technologies and their documented performance in managing background signals and enhancing sensitivity for pollutant detection.
Table 1: Comparison of SERS Substrate Performance for Environmental Sensing
| Substrate Type | Key Feature | Target Analyte(s) | Reported Limit of Detection (LOD) | Fluorescence & Background Handling |
|---|---|---|---|---|
| LB-AgNPs (Ultralow-Background) [68] | One-pot synthesis with inorganic halide ligands | Sulfonamide antibiotics, Organophosphorus pesticides | Not Specified | Ultralow background from reduced chemical residues; enables clear identification of degradation intermediates. |
| Graphene/Ag-Nanocube Hybrid [69] | Graphene adsorbs pollutants via Ï€-Ï€ stacking; Ag-NCs provide EM enhancement | DDT, Fluorene, Naphthalene | ~10â»â¸ - 10â»â¹ M | Graphene can quench fluorescence; composite design enhances analyte capture. |
| Au/CW-m (Cicada Wing) [70] | DC magnetron sputtering of Au on bio-template | Rhodamine-6G | 10â»â¸ M | Green fabrication minimizes chemical contaminants; structure may reduce background. |
| Dry-Deposited AuNPs on Quartz [71] | Solvent-free, gas-phase nanoparticle deposition | Caffeine | 1 ppm (in water) | Very low contamination substrate; flat baseline at 830 nm excitation reduces fluorescence. |
| 3D SERS Substrates [1] | Volumetric hot spot distribution (e.g., nanowires, porous frameworks) | Various biomarkers and analytes | Enhancement Factor >10⸠(theoretical) | Enhanced light trapping and analyte diffusion; can be designed with fluorescence-quenching materials. |
Experimental Protocols for Key Substrates
Protocol for Ultralow-Background AgNPs (LB-AgNPs)
This one-pot synthesis method aims to eliminate the high background signals typically caused by chemical residues from conventional wet-chemical synthesis [68].
- Synthesis: Inorganic halide ligands (e.g., chloride, bromide) are introduced during the reduction of silver ions (e.g., with sodium citrate). These ligands act as stabilizers, preventing the adsorption of organic residues from reducing agents onto the nanoparticle surface.
- Characterization: The resulting colloidal silver nanoparticles (AgNPs) are characterized by techniques like SEM and UV-Vis spectroscopy to confirm size, morphology, and plasmonic properties.
- SERS Measurement: The LB-AgNP colloid is mixed with the analyte solution (e.g., a pesticide solution). A droplet of the mixture is then dried on a substrate like silicon or glass before Raman measurement. Using a 780 nm excitation wavelength is reported to help reduce fluorescence interference [68].
Protocol for Dry-Deposited Gold Nanoparticle Substrates
This protocol focuses on using a commercially available, reproducible substrate to minimize variability [71].
- Substrate Preparation: Nikalyte SERS substrates, which consist of ultra-pure gold nanoparticles deposited on a quartz fiber pad via a solvent-free gas-phase process, are used. This method avoids chemical contaminants.
- Sample Application: Exactly 15 µL of the analyte solution (e.g., a series of caffeine dilutions in water) is pipetted onto one corner of the substrate's active area. The solution is allowed to spread and the substrate changes color from pink to blue, indicating agglomeration and readiness.
- SERS Measurement: Spectra are acquired immediately after the color change. It is critical to use a laser power density below 20 W/cm² (e.g., 80 mW over a ~900 µm spot) to avoid substrate damage or signal saturation. For fluorescence suppression, 830 nm excitation is recommended, as it provides a flatter baseline compared to 785 nm [71].
Protocol for Graphene/Ag-Nanocube Hybrid Substrates
This protocol leverages a hybrid material for both efficient pollutant capture and strong signal enhancement [69].
- Substrate Fabrication:
- Synthesis of Ag Nanocubes (Ag-NCs): Ag-NCs are synthesized via a polyol method using ethylene glycol as a solvent and reducing agent.
- Preparation of Graphene Oxide (GO): GO is prepared from expandable graphite using a modified Hummers' method.
- Assembly: The Ag-NCs and GO solution are mixed, and the hybrid nanostructure is formed through self-assembly, creating a film where Ag-NCs are evenly distributed on graphene sheets.
- SERS Detection:
- The hybrid film is immersed in a solution containing the target pollutant (e.g., DDT, naphthalene) for a set time, allowing the graphene to adsorb the molecules.
- The substrate is rinsed, dried, and then subjected to Raman analysis. The strong adsorption capacity of graphene concentrates the analyte molecules within the enhanced electromagnetic fields ("hot spots") generated by the Ag-NCs.
Visualizing Substrate Selection and Optimization Pathways
The following diagram outlines a strategic decision-making process for selecting and optimizing SERS substrates based on specific research goals related to background signal and fluorescence.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key Reagents and Materials for SERS Substrate Development and Analysis
| Item | Function in SERS Research | Examples / Notes |
|---|---|---|
| Noble Metal Precursors | Source for creating plasmonic nanostructures. | Chloroauric acid (HAuCl₄), Silver nitrate (AgNO₃). |
| Reducing & Stabilizing Agents | Control nucleation and growth of metal nanoparticles; critical for background levels. | Sodium citrate, ascorbic acid. Inorganic halides (e.g., KCl, NaBr) for low-background substrates [68]. |
| 2D Nanomaterials | Act as adsorption platforms; can quench fluorescence and provide chemical enhancement. | Graphene oxide (GO), reduced Graphene Oxide (rGO) [69]. |
| Biological or Synthetic Templates | Provide nanostructured scaffolds for depositing SERS-active metals. | Cicada wings [70], anodic aluminum oxide (AAO) membranes. |
| Model Analytic Probes | Used for standardizing and evaluating substrate performance. | Rhodamine 6G (R6G), Crystal Violet, Methylene Blue, Caffeine [71]. |
| Target Environmental Pollutants | The analytes of interest for detection. | Pesticides (DDT, thiram), pharmaceuticals, polycyclic aromatic hydrocarbons (PAHs) [69] [8]. |
| Portable Raman Spectrometers | Enable on-site analysis with flexible excitation wavelengths. | Systems with 785 nm and 830 nm lasers are valuable for fluorescence avoidance [71]. |
| 4-methyl-2-oxo-2H-chromen-7-yl sulfamate | 4-Methyl-2-oxo-2H-chromen-7-yl sulfamate|CAS 136167-05-0 | 4-Methyl-2-oxo-2H-chromen-7-yl sulfamate (CAS 136167-05-0) is a coumarin-sulfamate hybrid for antibacterial and anti-inflammatory research. For Research Use Only. Not for human or veterinary use. |
| (1R,2S)-1,2-dihydrophenanthrene-1,2-diol | (1R,2S)-1,2-dihydrophenanthrene-1,2-diol|High-Purity | (1R,2S)-1,2-dihydrophenanthrene-1,2-diol. A key PAH metabolite for studying carcinogenic pathways. For Research Use Only. Not for human use. |
The optimization of the signal-to-noise ratio in SERS for environmental monitoring is a multi-faceted challenge that requires a strategic approach to substrate selection and experimental design. Substrates fabricated via clean, solvent-free processes or designed with inorganic ligands effectively minimize the intrinsic chemical background, allowing the target pollutant's signal to dominate. For tackling fluorescence interference, the combination of longer-wavelength laser excitation (e.g., 830 nm) and the integration of fluorescence-quenching materials like graphene presents a robust solution. Furthermore, the transition from traditional 2D substrates to 3D architectures promises greater signal enhancement and reproducibility by maximizing the density of "hot spots." Researchers must align their choice of substrate and methodology with the specific nature of the pollutant and the environmental matrix to achieve reliable, high-sensitivity detection.
The detection and monitoring of environmental pollutants—ranging from pesticides and pharmaceuticals to heavy metals—represent a critical challenge in ensuring water safety and public health. [8] Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique to address this challenge, capable of providing ultrasensitive, fingerprint-based identification of pollutants. [2] [8] The analytical performance of a SERS sensor is fundamentally governed by its substrate, a nanostructured material that amplifies the inherently weak Raman signal via plasmonic effects. [72] [73] The fabrication route chosen to create these nanostructures directly dictates critical substrate properties such as sensitivity, reproducibility, cost, and suitability for real-world applications. [72] This guide provides a comparative evaluation of the three principal fabrication philosophies—top-down, bottom-up, and hybrid approaches—within the specific context of developing SERS sensors for environmental pollutant detection.
Core Fabrication Approaches: A Comparative Analysis
The creation of SERS-active nanostructures primarily follows two distinct paradigms: top-down, which involves the controlled decomposition or patterning of bulk material, and bottom-up, which relies on the assembly of atomic or molecular components into nanostructures. [72] A third category, the hybrid approach, seeks to synergize the strengths of both. The table below presents a systematic comparison of these methodologies.
Table 1: Comparison of SERS Substrate Fabrication Techniques for Environmental Sensing
| Feature | Top-Down Approach | Bottom-Up Approach | Hybrid Approach |
|---|---|---|---|
| Basic Principle | Patterning and etching bulk materials into nanostructures. [72] | Self-assembly of nanoparticles from atomic/molecular precursors. [72] | Combines top-down patterning with bottom-up assembly. [72] |
| Common Methods | Photolithography, Electron Beam Lithography (EBL). [72] | Colloidal synthesis, chemical reduction. [72] [8] | EBL with nano-transfer printing, templated assembly. [72] |
| Typical SERS Enhancement Factor (EF) | (10^4 - 10^7) (moderate). [72] | (10^8 - 10^{12}) (very high, especially in "hot spots"). [72] | Varies, aims to combine high EF with good uniformity. [72] |
| Reproducibility & Uniformity | High. Offers precise control over nanostructure geometry and placement. [72] | Low to Moderate. Challenging to control precise positioning and uniformity. [72] [73] | Improved. Aims for better reproducibility than pure bottom-up. [72] |
| Cost & Scalability | High cost, low throughput, difficult to scale up. [72] | Low cost, simpler, potential for large-scale production. [72] | Moderate cost, scalability depends on specific methods used. [72] |
| Best Use Cases in Environmental Detection | Quantitative analysis requiring high reproducibility. [72] | High-sensitivity screening for trace-level pollutants. [8] | Developing specialized, high-performance substrates. [72] |
The following workflow diagram illustrates the fundamental processes and decision points involved in selecting and implementing these fabrication strategies for SERS substrate development.
Experimental Protocols and Performance Data
The ultimate validation of any SERS substrate lies in its experimental performance in detecting target analytes. For environmental monitoring, this is typically quantified by the Limit of Detection (LOD), which is the lowest concentration of a pollutant that can be reliably detected. [8] The following table compiles experimental data from recent studies, highlighting the achieved LODs for various pollutants using substrates fabricated by different methods.
Table 2: Experimental SERS Performance in Pollutant Detection
| SERS Substrate | Fabrication Approach | Target Pollutant | Sample Matrix | Limit of Detection (LOD) | Ref. |
|---|---|---|---|---|---|
| Au@Ag Nanocuboids | Bottom-up (colloidal) | Malachite Green (dye) | Fishpond Water | ( 8.7 \times 10^{-10}) M | [8] |
| TiOâ‚‚/Ag Flower-like Nanomaterial | Hybrid | Malachite Green (dye) | Lake Water | ( 10^{-12}) M | [8] |
| AlOOH@Ag Nanostructures | Bottom-up | Congo Red (dye) | River & Industrial Wastewater | ( 10^{-9}) M | [8] |
| Defect-Graphene/Ag-MIP | Hybrid | p-Nitroaniline (PNA) | River Water | ( 2.5 \times 10^{-15}) M | [8] |
| Porous Au Supraparticles | Bottom-up (assembly) | Malachite Green Isothiocyanate | Wastewater Influent | ( 10^{-8}) M | [8] |
| Ag/ZIF-67/TiOâ‚‚/Cu | Hybrid | 4-Aminothiophenol (pesticide) | River Water | ( 5 \times 10^{-11}) M | [8] |
Detailed Experimental Protocol: Colloidal Ag Nanocube Synthesis for Dye Detection
A prominent example of a bottom-up protocol is the synthesis of silver nanocubes (AgNCs) for the detection of organic dyes like Malachite Green (MG) in water. [8]
- Substrate Fabrication: Silver nanocubes are synthesized through a polyol process, which involves the reduction of silver nitrate (AgNO₃) in ethylene glycol using polyvinylpyrrolidone (PVP) as a capping agent to control morphology. The nanocubes are then purified and assembled into a monolayer on a solid support.
- Analyte Exposure: Water samples (e.g., from aquaculture) are collected and filtered to remove large particulates. The sample is then incubated with the AgNC substrate, allowing MG molecules to adsorb onto the silver surface, particularly in the enhanced field regions ("hot spots").
- SERS Measurement: Raman spectra are acquired using a spectrometer equipped with a 785 nm laser, chosen because its energy matches the plasmon resonance peak of the AgNC monolayer, maximizing the electromagnetic enhancement. [8]
- Data Analysis: The characteristic fingerprint peaks of MG are identified. The LOD of ( 2.6 \times 10^{-7}) M was determined by measuring a series of standard solutions and calculating the concentration that gives a signal-to-noise ratio of 3. [8]
The Scientist's Toolkit: Essential Reagents and Materials
The fabrication and application of SERS substrates require a range of specialized materials. The table below lists key solutions and their functions in the context of substrate development and environmental sensing.
Table 3: Key Research Reagent Solutions for SERS Substrate Development
| Reagent / Material | Function in SERS Substrate Fabrication & Sensing |
|---|---|
| Noble Metal Salts (e.g., Chloroauric Acid, Silver Nitrate) | Precursors for the synthesis of plasmonic nanoparticles (Au, Ag) via bottom-up chemical reduction. [72] [8] |
| Polyvinylpyrrolidone (PVP) | A capping agent used in colloidal synthesis to control nanoparticle growth, stabilize dispersion, and prevent aggregation. [8] |
| Electron-Sensitive Resists (e.g., PMMA) | Essential polymers used in top-down Electron Beam Lithography (EBL) to create nanopatterns on substrates. [72] |
| Functional Materials (e.g., Graphene Oxide, ZIF-8 MOF) | Integrated into hybrid substrates to provide additional chemical enhancement, improve molecule adsorption, and increase stability against oxidation. [2] [8] |
| Molecular Imprinted Polymers (MIPs) | Synthetic receptors incorporated into substrates to provide high selectivity for specific pollutant molecules, reducing matrix interference. [8] |
| Calibrant Dyes (e.g., Rhodamine 6G) | Standard molecules with well-known Raman spectra used to calibrate SERS substrates and calculate Enhancement Factors (EFs). [27] |
The choice of fabrication technique for SERS substrates is a fundamental decision that involves balancing the often-competing demands of sensitivity, reproducibility, and cost. Top-down methods like EBL provide unparalleled control and are ideal for developing standardized, quantitative sensors. In contrast, bottom-up colloidal synthesis offers a straightforward path to ultra-sensitive substrates capable of detecting pollutants at trace concentrations, albeit with higher signal variance. The emerging trend of hybrid approaches represents a promising pathway forward, leveraging the strengths of both methods to create substrates that are both highly sensitive and reasonably reproducible. For researchers focused on environmental pollutants, the optimal fabrication strategy will ultimately depend on the specific application: high-precision top-down substrates for regulatory-grade quantification, and cost-effective, sensitive bottom-up substrates for widespread initial screening and monitoring of water quality.
Numerical Optimization and FEM Modeling for Substrate Design
Surface-enhanced Raman scattering (SERS) substrates are critical for detecting environmental pollutants (e.g., pesticides, pharmaceuticals) at trace concentrations. The design of these substrates relies on numerical optimization and finite element method (FEM) modeling to maximize enhancement factors (EFs) by engineering electromagnetic "hot spots" [1]. This guide compares the performance of 2D vs. 3D substrates, monometallic vs. bimetallic nanostructures, and computational approaches for optimizing substrate architecture. Experimental data and modeling protocols are provided to aid researchers in selecting and designing substrates for environmental sensing.
Performance Comparison of SERS Substrates
2D vs. 3D Substrates
3D substrates (e.g., nanowires, porous frameworks) outperform 2D planar structures by providing volumetric hot spots, improved analyte diffusion, and higher reproducibility. Key comparisons include [1]: Table 1: 2D vs. 3D SERS Substrates
| Feature | 2D Substrates | 3D Substrates |
|---|---|---|
| Hot Spot Distribution | Planar | Volumetric |
| Enhancement Factor (EF) | 10âµâ€“10â· | >10⸠|
| Reproducibility | Moderate | High (RSD < 10%) |
| Analyte Accessibility | Limited diffusion | Enhanced porous diffusion |
| Fabrication Methods | Lithography, self-assembly | Template growth, freeze-drying |
Applications: 3D substrates enable detection of pollutants like uranyl ions (UO₂²âº) at 0.838 pM, leveraging dense hot spots and fluid transport [1].
Monometallic vs. Bimetallic Substrates
Bimetallic Au-Ag nanoparticles on reduced graphene oxide (rGO) exhibit synergistic plasmonic coupling, achieving higher EFs than monometallic counterparts [74]: Table 2: Substrate Composition and EFs
| Substrate Type | EF (Rhodamine 6G) | Detection Limit |
|---|---|---|
| Au/rGO | 2.70 × 10â· | ~10â»â¹ M |
| Ag/rGO | 4.92 × 10â· | ~10â»â¹ M |
| Au-Ag/rGO | 1.12 × 10⸠| 10â»Â¹â° M |
Mechanism: Bimetallic NPs enhance localized surface plasmon resonance (LSPR), generating stronger electromagnetic fields at nanogaps [74].
Experimental Protocols for Substrate Evaluation
Substrate Fabrication and Functionalization
Bimetallic Au-Ag/rGO Synthesis:
Cellulose-Based Flexible Substrates:
- Functionalize nanocellulose films with Au/Ag NPs using in-situ reduction or adsorption.
- Advantages: Biodegradability, low background signal, and adaptability to irregular surfaces [27].
SERS Measurement and EF Calculation
Analyte Preparation:
EF Calculation:
- Apply the formula: ( AEF = \frac{I{SERS}}{I{Raman}} \times \frac{C{Raman}}{C{SERS}} ) where ( I ) = peak intensity (e.g., 1358 cmâ»Â¹ for Rhodamine B) and ( C ) = concentration [12].
Instrumentation:
Numerical Optimization and FEM Modeling
Computational Approaches
- Objective Function: Replace local field enhancement (( |E|^4 )) with the Purcell factor to account for 3D field inhomogeneity and radiative efficiency [75].
- FEM Workflow:
Key Modeling Insights
- Hot Spot Engineering: Nanogaps (1–2 nm) in Au-Ag NP dimers increase EF by 10²–10ⴠcompared to isolated NPs [74].
- 3D Substrates: Vertical nanostructures (e.g., nanopillars) enhance light trapping and signal uniformity [1].
Diagram 1: FEM-Based Substrate Optimization Workflow
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagents for SERS Substrate Development
| Reagent/Material | Function |
|---|---|
| Au/Ag Nanoparticles | Plasmonic enhancement via LSPR; Au offers stability, Ag higher EF [74]. |
| Reduced Graphene Oxide (rGO) | Provides 2D substrate for NP attachment; quenches fluorescence [74]. |
| Rhodamine 6G/B | Raman reporter for EF calibration [74] [12]. |
| Cellulose Films | Flexible, eco-friendly substrate with low background interference [27]. |
| Propranolol/Malachite Green | Model pollutants for environmental detection [76] [77]. |
Numerical optimization and FEM modeling are indispensable for designing high-performance SERS substrates. 3D and bimetallic architectures consistently outperform traditional 2D and monometallic designs, offering EFs >10⸠and detection limits down to 10â»Â¹â° M. Integrating experimental data with computational models enables precise control over hot spots, advancing environmental pollutant monitoring. Future directions include stimuli-responsive designs and machine learning-driven optimization [1].
Benchmarking SERS Performance: Validation, Comparative Analysis, and Standardization
Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique for detecting environmental pollutants at ultratrace concentrations. The performance of SERS-based sensing platforms depends critically on the properties of the substrate, which amplifies the inherently weak Raman signal through plasmonic effects. Two key metrics for evaluating substrate performance are the Enhancement Factor (EF), which quantifies signal amplification, and the Limit of Detection (LOD), which defines the lowest detectable analyte concentration. This guide provides a systematic comparison of EF and LOD values across various SERS substrate technologies, focusing on their application for environmental pollutant detection. The analysis synthesizes recent experimental data to inform substrate selection and development for researchers and scientists working in environmental monitoring and analytical chemistry.
Performance Comparison of SERS Substrates
The following table summarizes the quantitative performance of various SERS substrates reported in recent literature, providing a direct comparison of their Enhancement Factors and Limits of Detection for specific probe molecules and environmental analytes.
Table 1: Performance Comparison of SERS Substrates for Environmental Detection
| Substrate Type | Probe Molecule/Analyte | Enhancement Factor (EF) | Limit of Detection (LOD) | Key Features |
|---|---|---|---|---|
| Bimetallic Au-Ag/rGO [74] | Rhodamine 6G (R6G) | (1.12 \times 10^{8}) | (10^{-10}) M | Synergistic plasmonic coupling, high hotspot density |
| Au Nanostars [78] | Rhodamine B (RhB) | (3.57 \times 10^{6}) | Not Specified | Sharp tips for strong field enhancement |
| Ag-NPs (High Concentration) [79] | Methylene Blue (MB) | (1.13 \times 10^{5}) | (0.5 \times 10^{-6}) M | Cluster formation creates more "hot spots" |
| Ag/PMMA Composites [79] | Methylene Blue (MB) | (1.70 \times 10^{4}) | (2.5 \times 10^{-6}) M | Polymer provides mechanical stability, but can shield EM fields |
| Hydrophobic CuO@Ag Nanowire [80] | 50 nm Polystyrene Nanoplastics | Not Specified | (10^{-10}) wt% (Nanoplastics) | Utilizes coffee-ring effect for preconcentration |
| Gold Pyramid Array [81] | Not Specified | ( \sim 10^{8}) (Simulated) | Not Specified | High reproducibility via silicon mold fabrication |
The data reveals that composite materials, particularly bimetallic nanoparticles integrated with graphene derivatives, achieve the highest performance. The bimetallic Au-Ag/rGO substrate demonstrates a superior EF of (1.12 \times 10^{8}), which is 2 to 4 times higher than its monometallic counterparts [74]. This is attributed to the synergistic effect between Au (chemical stability) and Ag (strong plasmonic properties), combined with the large surface area and chemical adsorption properties of rGO. Substrates with sharp morphological features, such as Au nanostars and simulated gold pyramids, also show high EFs ((10^{6}-10^{8})) due to the lightning rod effect and concentrated electromagnetic fields at their tips [78] [81]. Simpler structures like Ag-NPs and Ag/PMMA composites show more modest but still substantial EFs ((10^{4}-10^{5})), sufficient for many practical applications [79].
Detailed Experimental Protocols
To ensure the reproducibility of SERS performance metrics, the following section details the key experimental methodologies employed for fabricating and characterizing the high-performing substrates listed above.
Fabrication of Bimetallic Au-Ag/rGO Substrates
The synthesis of the high-performance Au-Ag/rGO substrate involves a multi-step, bottom-up chemical approach [74]:
- Synthesis of Graphene Oxide (GO): GO is first synthesized from graphite using a modified Hummer's method.
- Thermal Reduction to rGO: The GO is thermally reduced to form rGO, which provides a wrinkled, two-dimensional scaffold for nanoparticle attachment.
- Nanoparticle Synthesis: Monometallic (Au, Ag) and bimetallic (Au-Ag) nanoparticles are synthesized separately via chemical reduction of their metal salt precursors.
- Formation of Nanocomposite: The as-synthesized nanoparticles are combined with the rGO suspension to form the final nanocomposites (Au/rGO, Ag/rGO, and Au-Ag/rGO). The functional groups on the rGO surface facilitate the binding and uniform distribution of the nanoparticles, preventing agglomeration.
Electron Beam Lithography of Gold Nanostructures
For precisely controlled nanostructures, Electron Beam Lithography (EBL) offers a top-down alternative [78]:
- Substrate Cleaning: A SiOâ‚‚/Si substrate is meticulously cleaned in an ultrasonic bath with acetone, ethanol, and deionized water.
- Resist Patterning: The substrate is coated with a resist, and the desired nanostructure arrays (e.g., circles, triangles, stars) are written using an electron beam lithography system.
- Metal Deposition and Lift-Off: A thin adhesion layer of Cr (5 nm) followed by a Au layer (10-15 nm) is deposited via electron beam evaporation. Subsequently, a lift-off process removes excess metal, leaving behind the precise gold nanostructure arrays on the substrate.
SERS Measurement and EF Calculation Protocol
A standardized protocol is critical for meaningful EF comparisons across studies. The analytical enhancement factor is commonly calculated as follows [78]:
- Spectra Acquisition: SERS spectra ((I{SERS})) are collected from the analyte (e.g., Rhodamine B at (10^{-6}) mol/L) adsorbed on the SERS substrate. Reference Raman spectra ((I{REF})) are obtained from a high concentration (e.g., 0.1 mol/L) of the same analyte on a non-enhancing substrate (e.g., plain SiOâ‚‚/Si).
- EF Calculation: The EF is calculated using the formula: (EF = \frac{I{SERS}}{I{REF}} \times \frac{C{REF}}{C{SERS}}) where (C{REF}) and (C{SERS}) are the analyte concentrations used for the reference and SERS measurements, respectively [78].
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful SERS substrate development relies on a set of core materials and reagents, each serving a specific function in the fabrication and sensing process.
Table 2: Essential Reagents and Materials for SERS Substrate Development
| Material/Reagent | Function in SERS Substrate Development | Example Use Cases |
|---|---|---|
| Gold (Au) & Silver (Ag) Salts | Precursors for synthesizing plasmonic nanoparticles (e.g., from HAuCl₄, AgNO₃). | Chemical synthesis of Au, Ag, and Au-Ag nanoparticles [74] [79]. |
| Reduced Graphene Oxide (rGO) | Provides a 2D platform for nanoparticle support, prevents aggregation, and contributes chemical enhancement via charge transfer. | Au-Ag/rGO composites for ultra-sensitive R6G detection [74]. |
| Poly(methyl methacrylate) (PMMA) | A polymer used to create flexible, stable composite substrates or microsphere opals. | Ag/PMMA composite substrates for analyte detection [79]. |
| Silicon Wafers | A common, flat, and easily functionalized solid support for fabricating SERS substrates. | Base substrate for EBL-fabricated nanostructures [78] and pyramid arrays [81]. |
| Rhodamine Dyes (R6G, RhB) | Standard probe molecules with well-known Raman spectra used to benchmark and calculate SERS EFs. | Quantifying EF for nanostructures [78] and nanocomposites [74]. |
| Sodium Citrate & Sodium Borohydride | Common reducing and stabilizing agents in the wet-chemical synthesis of metal nanoparticles. | Synthesis of spherical Ag-NPs and Au-NPs [74] [79]. |
Workflow and Signaling Pathways
The process of developing and applying a SERS substrate for environmental detection involves a sequence of key steps, from design to quantitative analysis. Furthermore, the signal enhancement is governed by the interplay of distinct physical mechanisms.
SERS Substrate Development and Enhancement Workflow
The SERS enhancement mechanism is primarily a combination of two effects [2]. The Electromagnetic Mechanism (EM) is the dominant contributor, arising when incident light excites localized surface plasmon resonance (LSPR) in noble metal nanostructures. This creates intensely amplified electromagnetic fields, particularly in nanoscale gaps known as "hot spots." The total EM enhancement is proportional to the fourth power of the local field intensity ((|E|^4)) [74] [81]. The Chemical Mechanism (CM) involves a charge-transfer process between the analyte molecule and the substrate surface, which modifies the molecular polarizability and provides additional signal enhancement. In composite substrates, materials like graphene oxide can significantly boost this chemical component [24] [74].
The accurate detection of environmental pollutants is paramount for protecting public health and ensuring ecosystem safety. Within this field, analytical techniques must balance sensitivity, specificity, speed, and cost-effectiveness to be effective for both monitoring and research. Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique that leverages nanostructured substrates to significantly amplify the Raman scattering signal of target molecules, enabling their identification at ultra-low concentrations [52]. This review provides a objective, data-driven comparison between SERS and three well-established workhorses of the analytical laboratory: High-Performance Liquid Chromatography (HPLC), Gas Chromatography-Mass Spectrometry (GC-MS), and Enzyme-Linked Immunosorbent Assay (ELISA). The evaluation is framed within the context of validating SERS substrates for the detection of environmental pollutants, including pesticides, mycotoxins, and other chemical contaminants, providing researchers with a clear understanding of the capabilities and limitations of each platform.
Surface-Enhanced Raman Spectroscopy (SERS)
SERS operates on the principle of enhancing the inherently weak Raman scattering of molecules adsorbed onto or in close proximity to specially engineered metallic nanostructures. The core enhancement mechanisms are:
- Electromagnetic Enhancement (EM): The primary enhancement mechanism, resulting from the excitation of localized surface plasmon resonances (LSPR) on nanostructured noble metal surfaces (e.g., Ag, Au). This creates intensely localized electromagnetic fields, known as "hot spots," which can enhance Raman signals by factors of up to 10^10 or more [35] [39].
- Chemical Enhancement (CE): A secondary mechanism involving charge transfer between the analyte molecule and the metal surface, which can alter the polarizability of the molecule and further amplify its Raman signal [35].
SERS is valued for its rapid analysis times, minimal sample preparation, "fingerprint" identification capability, and potential for single-molecule sensitivity [39] [52].
High-Performance Liquid Chromatography (HPLC) & Liquid Chromatography-Mass Spectrometry (LC-MS)
HPLC separates compounds in a liquid mixture based on their differential partitioning between a mobile liquid phase and a stationary phase. When coupled with mass spectrometry (LC-MS), it also provides identification and confirmation based on mass-to-charge ratios. These methods are considered gold standards for quantitative analysis due to their high sensitivity, accuracy, and ability to separate and quantify multiple analytes simultaneously [82] [83]. The main drawbacks include high instrument costs, complex operation, and time-consuming sample preparation [39].
Gas Chromatography-Mass Spectrometry (GC-MS)
GC-MS combines gas chromatography, which separates volatile compounds, with mass spectrometry for detection. It is ideal for volatile and semi-volatile organic compounds, offering high sensitivity and definitive structural identification [82]. A significant limitation is that non-volatile or thermally labile analytes often require derivatization—a chemical modification step to increase their volatility—before analysis [82].
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA is an immunoassay that uses antibodies immobilized on a plate to capture specific target antigens. Detection is achieved through an enzyme-linked antibody that produces a measurable signal, typically a color change. Its key advantages are high throughput, cost-effectiveness, and simplicity, making it excellent for screening [84] [82]. However, it can suffer from cross-reactivity with similar compounds and generally provides less specific, quantitative data compared to chromatographic methods [82] [39].
Comparative Performance Analysis
The following tables summarize the key operational characteristics and performance data of the four analytical platforms based on documented applications in environmental analysis.
Table 1: Operational Characteristics of Analytical Platforms
| Feature | SERS | HPLC/LC-MS | GC-MS | ELISA |
|---|---|---|---|---|
| Principle | Vibrational Spectroscopy (Signal Enhancement) | Chromatography & Mass Spectrometry | Chromatography & Mass Spectrometry | Immunoassay (Antibody Binding) |
| Sensitivity | Sub-μg/L to μg/L range [35] | Parts per billion (ppb) or lower [82] | High (ppt-ppb) [82] | High (nanogram levels) [82] |
| Selectivity/Specificity | Molecular "Fingerprint" | High (Separation + Mass ID) | High (Separation + Mass ID) | High, but potential for cross-reactivity [82] |
| Sample Throughput | Rapid (Minutes) [39] | Slow (Can be hours) [82] | Slow (Can be hours) | High (Batch analysis) [82] |
| Sample Preparation | Minimal | Complex, time-consuming [39] | Complex, may require derivatization [82] | Relatively simple [84] |
| Multiplexing Capability | Good for multiple analytes [39] | Excellent (Multi-residue) | Excellent (Multi-residue) | Limited (Typically single-analyte) |
| Quantitative Accuracy | Good (Improving with standardization) | Excellent (Gold standard) [82] | Excellent (Gold standard) | Good (Semi-quantitative) [82] |
| Instrument Cost | Moderate-High | High [39] | High [82] | Low [82] |
| Portability | Yes (Emerging systems) [35] | No | No | Yes (Lateral flow formats) [82] |
Table 2: Documented Performance in Contaminant Detection
| Analyte Class | Specific Analyte | Technique | Reported LOD/LOQ | Sample Matrix | Citation |
|---|---|---|---|---|---|
| Mycotoxin | Ochratoxin A (OTA) | HPLC-FLD | LOQ: 0.05 μg/L | Human Blood Serum | [84] |
| Mycotoxin | Ochratoxin A (OTA) | ELISA | LOQ: 0.05 μg/L | Human Blood Serum | [84] |
| Pesticide | Organophosphorus Pesticides (OPPs) | SERS | Sub-μg/L to low μg/L | Food Matrices, Water | [35] |
| Pesticide | Organophosphorus Pesticides (OPPs) | GC-MS / HPLC | - | Food, Environment | [82] [35] |
| Antibiotic | Chloramphenicol | ELISA, HPLC, GC-MS | - | Seafood, Meat, Honey | [85] |
| Cyanotoxin | Microcystins (MCs) | ELISA, HPLC-MS | e.g., 0.2–200 ng/mL (ELISA) | Water | [83] |
Experimental Protocols for Cross-Platform Validation
Protocol: HPLC-FLD vs. ELISA for Mycotoxin Analysis
A direct comparison of ELISA and HPLC for determining Ochratoxin A (OTA) in human blood serum highlights a standard validation workflow [84] [86].
- Sample Preparation: Serum samples are processed, often involving dilution and extraction with organic solvents like methanol. For HPLC, a more extensive clean-up and concentration step may be required.
- Analysis:
- HPLC-FLD: The reference method. Separation is performed on a reversed-phase C18 column. OTA is detected and quantified based on its native fluorescence, with validation according to protocols like those from AOAC [84].
- ELISA: A competitive immunoassay where samples are incubated in antibody-coated wells. The intensity of the resulting colorimetric signal is inversely proportional to the OTA concentration.
- Data Correlation: Results from both methods are statistically correlated. The cited study of 115 serum samples found a very good correlation coefficient (r = 0.907), though ELISA tended to underestimate OTA at very low concentrations [84].
Protocol: SERS Workflow for Pesticide Detection
The typical workflow for SERS-based detection of pollutants like organophosphorus pesticides (OPPs) involves [35] [39]:
- Substrate Engineering: Fabricating and characterizing plasmonic nanostructures (e.g., Ag or Au nanoparticles, nanostars, or bimetallic hybrids) that provide a high density of SERS "hot spots."
- Sample Preparation and Exposure: For liquid samples (e.g., juice, agricultural water), minimal preparation such as filtration or centrifugation is performed. The sample is then brought into contact with the SERS substrate, allowing target molecules to adsorb to the surface.
- SERS Measurement: The substrate is irradiated with a laser, and the inelastically scattered Raman light is collected to generate a fingerprint spectrum.
- Signal Interpretation: Characteristic peaks of the target pesticide (e.g., from P=O or P=S functional groups) are identified. Quantification is achieved by measuring the intensity of these peaks, often using calibration curves. Advanced studies are employing machine learning to improve spectral interpretation and quantification accuracy [35].
Technical Considerations and Research Gaps
Advantages and Limitations in Practice
- SERS vs. Chromatography/MS: While HPLC and GC-MS provide unparalleled quantitative accuracy and are recognized as confirmatory methods, they are laboratory-bound, expensive, and slow. SERS offers a path toward rapid, on-site screening but currently faces challenges with quantitative reproducibility and substrate batch-to-batch variability [35] [39]. Furthermore, SERS signal is highly dependent on the molecule's affinity for the substrate, which can be weak for some contaminants like organochlorine pesticides [39].
- SERS vs. ELISA: Both techniques are suitable for screening. ELISA benefits from well-established, commercial kits and high throughput. However, SERS possesses a key advantage: the ability to detect multiple, structurally diverse analytes simultaneously without cross-reactivity due to its fingerprinting capability, whereas ELISA kits are typically designed for a single analyte or a narrow class [39].
- Hybrid SERS Biosensors: A promising development is the integration of SERS with biological recognition elements like aptamers or antibodies. This creates a SERS biosensor that combines the high specificity of the bio-element with the sensitive, fingerprint detection of SERS, mitigating issues of non-specific adsorption and improving selectivity for complex matrices [39].
Critical Research Gaps
- Standardization: There is a critical lack of standardized protocols for SERS substrate fabrication and measurement procedures, hindering direct inter-laboratory comparisons [35].
- Matrix Effects: Robust methods to mitigate signal suppression or interference from complex environmental matrices (e.g., soil extracts, humic acids in water) require further development [35] [52].
- Quantitative Robustness: Improving the reproducibility and accuracy of SERS for reliable quantification, potentially through internal standards and advanced data processing, remains a primary research focus [35].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Reagent Solutions for Featured Techniques
| Item | Function/Description | Typical Application |
|---|---|---|
| Noble Metal Nanoparticles (Ag, Au) | Serve as the plasmonically active SERS substrate, generating the enhancement. Morphology (spheres, stars, rods) is critical. | SERS Substrate Engineering [35] [52] |
| Immunoaffinity Columns | Contain immobilized antibodies for highly specific extraction and clean-up of a target analyte from a complex sample matrix. | Sample Preparation for HPLC/LC-MS [82] [83] |
| C18 Solid-Phase Extraction (SPE) Cartridges | Reversed-phase sorbent for general-purpose extraction, clean-up, and concentration of semi-polar to non-polar analytes. | Sample Preparation for Chromatography [84] [83] |
| Commercial ELISA Kit | Provides pre-coated plates, antibodies, buffers, and standards for a ready-to-use, standardized assay for a specific analyte. | High-Throughput Screening [84] [82] |
| Derivatization Reagents | Chemicals used to modify non-volatile analytes (e.g., silylating agents) to make them volatile and stable for GC-MS analysis. | Sample Preparation for GC-MS [82] |
| Molecular Recognition Agents (Aptamers/Antibodies) | Provide high-affinity binding to specific targets; used to functionalize SERS substrates or nanoparticles to create selective biosensors. | SERS Biosensor Development [39] |
Visualized Workflows and Logical Relationships
The following diagrams illustrate the core working principles of SERS and a generalized experimental workflow for the cross-validation of analytical methods.
This cross-platform validation demonstrates that SERS, HPLC, GC-MS, and ELISA each occupy a distinct and valuable niche in the environmental pollutant detection landscape. HPLC and GC-MS remain the undisputed gold standards for sensitive, multi-residue, and definitive confirmatory analysis. ELISA provides an excellent, high-throughput solution for targeted screening. SERS has firmly established itself as a powerful technique offering unique advantages in speed, fingerprint identification, and potential for on-site analysis, particularly when configured as a biosensor. The future of the field lies not in one technique supplanting the others, but in their synergistic use—for example, using SERS for rapid, on-site screening and following up with LC-MS for confirmatory analysis—supported by continued research to standardize and robustify SERS technology for routine application.
Assessing Sensitivity, Specificity, and Robustness Across Pollutant Classes
Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique for detecting environmental pollutants, combining the molecular fingerprint specificity of Raman spectroscopy with significant signal amplification. This capability enables the identification and quantification of trace-level contaminants in complex matrices. The performance of SERS-based detection hinges critically on the properties of the substrate, which serves as the platform for signal enhancement. This guide provides a systematic comparison of SERS substrate performance across different pollutant classes, evaluating key parameters including sensitivity, specificity, and robustness to inform substrate selection for environmental monitoring applications.
The fundamental principle of SERS involves the dramatic amplification of Raman scattering signals from molecules adsorbed onto or in close proximity to nanostructured metallic surfaces. The enhancement arises from two primary mechanisms: the electromagnetic enhancement (EM), resulting from localized surface plasmon resonance (LSPR) in noble metal nanostructures, and the chemical enhancement (CM), involving charge transfer between the analyte and substrate [2]. The EM mechanism is generally considered the dominant contributor, capable of enhancing signals by factors of up to 10^10 to 10^12, theoretically enabling single-molecule detection [52] [2]. This extraordinary sensitivity, coupled with the technique's ability to provide unique molecular fingerprints, makes SERS particularly valuable for environmental analysis where pollutants often exist at ultra-trace concentrations amidst complex sample matrices.
SERS Enhancement Mechanisms and Substrate Design Principles
Fundamental Enhancement Mechanisms
The exceptional sensitivity of SERS stems from the synergistic interplay of two primary enhancement mechanisms. The electromagnetic enhancement (EM) is the dominant contributor, arising from the excitation of localized surface plasmon resonance (LSPR) in noble metal nanostructures. When incident light resonates with the collective oscillation of conduction electrons in metals like gold and silver, it generates intensely localized electromagnetic fields, particularly at sharp features, tips, and narrow gaps (known as "hot spots") [35] [2]. The Raman signal enhancement is approximately proportional to the fourth power of the localized electric field intensity (|E|^4), explaining why substrates with abundant hot spots can achieve enormous enhancement factors [52] [35].
The chemical enhancement (CM) mechanism involves charge transfer between the analyte molecules and the substrate surface, which alters the polarizability of the molecules and increases their Raman scattering cross-section [35] [2]. While typically contributing a lesser degree of enhancement (10-100 fold) compared to the EM mechanism, CM is highly dependent on the specific chemical interaction between the analyte and the substrate surface. For molecules directly chemisorbed to the metal surface, this mechanism can significantly complement the overall SERS effect.
Figure 1: SERS Enhancement Mechanisms. The electromagnetic enhancement (EM) pathway involves Localized Surface Plasmon Resonance (LSPR) creating "hot spots" with intensified fields. The chemical enhancement (CM) pathway involves analyte adsorption and charge transfer increasing molecular polarizability.
Strategic Substrate Functionalization for Environmental Targets
Most environmental pollutants lack strong intrinsic affinity for bare SERS substrates, necessitating strategic surface functionalization to bring target molecules within the short-range enhancement zone (typically within 5 nm) [41]. Key functionalization strategies include:
Electrostatic and Hydrophobic Interactions: Modifying substrate surface charge or hydrophobicity to enhance attraction to specific pollutants. For instance, aliphatic amino acids can create substrates with controllable surface charges ranging from -60 to +30 mV, reducing electrostatic repulsion with target analytes [41]. Similarly, alkyl dithiol modifications enhance affinity for hydrophobic pesticides like organochlorines, achieving detection limits down to 10^-8 mol L^-1 [41].
Surface Complexation: Immobilizing molecules that form specific complexes with target pollutants. For mercury detection, tryptophan-modified gold nanomaterials form complexes with Hg(II), enabling recognition at 5 ppb levels [41]. For trinitrotoluene (TNT), cysteine-modified gold nanoparticles form Meisenheimer complexes, achieving exceptional sensitivity down to 2 pico molar level [41].
Host-Guest Chemistry and Biorecognition Elements: Utilizing functionalized molecules with specific molecular recognition capabilities, such as cyclodextrins for aromatic compounds or aptamers/antibodies for high-specificity detection [41] [65]. These approaches are particularly valuable for detecting pollutants in complex environmental matrices where selectivity is crucial.
Comparative Performance Across Pollutant Classes
The effectiveness of SERS substrates varies significantly across different classes of environmental pollutants, depending on their chemical properties, affinity for the substrate, and the complexity of the sample matrix. The following analysis compares substrate performance for major pollutant categories.
Table 1: SERS Substrate Performance for Organic Pollutant Detection
| Pollutant Class | Example Analytes | Substrate Type | Functionalization | LOD | Enhancement Factor | Key Challenges |
|---|---|---|---|---|---|---|
| Organophosphorus Pesticides | Parathion, Malathion | Au-Ag nanostars, MoS2 nanoclusters | Graphene oxide, antibodies | sub-μg L^-1 to low μg L^-1 [35] | 10^6 - 10^9 [35] | Matrix interference in food samples [35] [65] |
| Polycyclic Aromatic Hydrocarbons (PAHs) | Naphthalene, Benzopyrene | Thiol-functionalized magnetic NPs | Alkyl chains for hydrophobic interaction | 10^-7 mol L^-1 [41] | Not specified | Weak affinity to bare substrates [41] |
| Synthetic Dyes | Rhodamine B, Crystal Violet | Commercial Au nanostructures, 1T/2H-MoS2 | None (direct adsorption) | 10^-12 M (RhB) [12] | 1.02 × 10^9 (1T/2H-MoS2) [87] | Fluorescence background [12] |
| Pharmaceuticals | Antibiotics, Antiepileptics | Ag NPs on regenerated cellulose | Molecularly imprinted polymers [88] | Variable by compound | Not specified | Complex biological matrices [88] |
Table 2: SERS Substrate Performance for Inorganic and Particulate Pollutant Detection
| Pollutant Class | Example Analytes | Substrate Type | Functionalization | LOD | Enhancement Factor | Key Challenges |
|---|---|---|---|---|---|---|
| Heavy Metals | Hg(II), Pb(II), U(VI) | Au NPs, functionalized substrates | DNAzymes, tryptophan, phosphonic acids | 0.2 ppt (Hg²âº) [41] | Not specified | Indirect detection often required [41] |
| Microplastics/Nanoplastics | Polystyrene, PET | Au NPs, Ag nanostars @ anodized aluminum oxide | None (direct adsorption) | 10 μg/mL (PS) [52] 0.05 mg/g [52] | Not specified | Low signal for pure polymers [52] |
| Gaseous Pollutants | Inorganic gases | Roughened electrodes, porous substrates | Specific capture agents | Variable by compound | Not specified | Sampling and preconcentration [41] |
Performance Analysis by Pollutant Class
Organic Pollutants: SERS demonstrates exceptional sensitivity for organic pollutants with inherent affinity for metal surfaces or those that can be functionalized for selective capture. Organophosphorus pesticides containing P=O and P=S groups show strong SERS activity due to semi-covalent chemisorption bonds with metal surfaces, resulting in significant chemical enhancement effects [35]. Substrates incorporating graphene oxide benefit from additional π-π interactions with aromatic rings in pesticide molecules, further improving sensitivity and enrichment capabilities [65]. The integration of biorecognition elements like antibodies and aptamers has significantly enhanced selectivity for specific organic pollutants in complex matrices such as food extracts and agricultural runoff [65].
Inorganic Pollutants: Heavy metal ions typically exhibit weak direct SERS activity, necessitating indirect detection strategies using reporter molecules that undergo spectral changes upon metal complexation [41]. For example, thymine-rich single-stranded DNA functionalized substrates can detect Hg²⺠at concentrations as low as 0.2 ppt through the formation of thymine-Hg²âº-thymine complexes that induce hairpin structural changes [41]. Similarly, DNAzyme-based substrates enable specific Pb²⺠detection with high sensitivity. These functionalized substrates must maintain performance under challenging environmental conditions, including low pH and high salt concentrations encountered in contaminated waters [41].
Particulate Pollutants: Microplastics and nanoplastics represent an emerging application for SERS, where substrates can be engineered to concentrate and identify polymer particles from environmental samples. The detection sensitivity for particulates depends heavily on effective preconcentration strategies and the intrinsic Raman cross-sections of the polymer materials [52].
Experimental Protocols and Methodologies
Standardized SERS Substrate Characterization
Comprehensive characterization of SERS substrates is essential for meaningful performance comparison across studies. Standard experimental protocols include:
Substrate Fabrication and Morphological Analysis:
- SEM Imaging: High-resolution scanning electron microscopy reveals nanostructure morphology, feature sizes, and interstructural distances. For example, comparative SEM analysis of commercial substrates shows fractal structures with particle sizes ranging from 100-300 nm (Substrate A), more ordered nanostructures with ~97 nm average size (Substrate B), and evenly distributed tiny nanoparticles of ~18 nm (Substrate C) [12].
- Enhancement Factor Calculation: The analytical enhancement factor (AEF) is calculated using the formula:
AEF = (I_SERS/I_Raman) × (C_Raman/C_SERS)where ISERS and IRaman are the measured intensities of a specific Raman peak with and without enhancement, while CRaman and CSERS are the corresponding analyte concentrations [12].
SERS Performance Evaluation:
- Rhodamine B Testing: A standard protocol involves preparing Rhodamine B solutions across a concentration range (e.g., 10^-2 M to 10^-12 M), immersing substrates for 1 hour, drying for 15 minutes, then acquiring spectra with multiple measurements (15-20 points) to account for signal heterogeneity [12].
- Mapping and Reproducibility Assessment: Spatial mapping across the substrate surface quantifies signal uniformity, with commercial photonic crystal substrates achieving relative standard deviations of ~3% across a 4×4 mm area [57].
Figure 2: SERS Substrate Evaluation Workflow. Standardized protocol for comprehensive SERS substrate assessment, from sample preparation through data analysis.
Advanced Complementarity Techniques
Theoretical Modeling: Finite Element Method (FEM) modeling in platforms like COMSOL Multiphysics provides valuable insights into electromagnetic field distribution and enhancement mechanisms. By importing actual SEM images of substrates to create geometrically accurate models, researchers can simulate local electric field enhancements and predict SERS performance, complementing experimental findings [12].
Portable System Integration: For field-based environmental monitoring, SERS substrates are increasingly evaluated using portable Raman systems with lower-power lasers (e.g., 532 nm diode-pumped laser at 2.55 mW) and simplified optics [12] [57]. These systems enable rapid detection (10-second acquisitions) while maintaining sensitivity to physiologically or environmentally relevant concentrations [57].
The Researcher's Toolkit: Essential Materials and Reagents
Table 3: Essential Research Reagents for SERS-Based Environmental Detection
| Category | Specific Examples | Function/Purpose | Application Notes |
|---|---|---|---|
| Plasmonic Materials | Gold nanoparticles (AuNPs), Silver nanoparticles (AgNPs) [65] | Provide electromagnetic enhancement via LSPR | AuNPs offer higher stability; AgNPs provide greater enhancement but can oxidize [2] |
| Functionalization Agents | Aliphatic amino acids, alkyl dithiols, DNA aptamers, antibodies [41] [65] | Enhance selectivity and analyte capture | Choice depends on target pollutant properties and required specificity [41] |
| Reference Analytes | Rhodamine B, Crystal Violet, 4-aminothiophenol [12] [87] | Substrate performance benchmarking | Enable standardized comparison across different substrate types [12] |
| Semiconductor Components | 1T/2H-MoS2 nanoclusters, graphene oxide [87] [65] | Provide chemical enhancement and improved adsorption | Particularly effective for aromatic pollutants via π-π interactions [87] [65] |
| Magnetic Components | Fe₃O₄ nanoparticles [65] | Enable sample concentration and separation | Facilitate analyte preconcentration from large sample volumes [65] |
| Support Matrices | Photonic crystals, anodized aluminum oxide, silicon wafers [12] [57] | Provide structured support for nanostructures | Engineered substrates offer superior reproducibility (<5% RSD) [57] |
The assessment of SERS substrates for environmental pollutant detection reveals a complex performance landscape where sensitivity, specificity, and robustness must be balanced according to application requirements. Noble metal substrates with tailored nanostructures currently provide the highest electromagnetic enhancement factors, while functionalized substrates incorporating molecular recognition elements address selectivity challenges in complex matrices. Emerging semiconductor-plasmonic hybrids like 1T/2H-MoS2 nanoclusters demonstrate exceptional enhancement factors up to 10^9, representing promising alternatives to traditional noble metals.
Critical gaps remain in standardization for quantitative analysis, with substrate reproducibility and signal uniformity continuing to challenge widespread implementation. Future developments should focus on engineered substrates with controlled architecture, multifunctional surfaces for broad-spectrum pollutant capture, and integration with portable analytical systems for field-deployable environmental monitoring solutions. As SERS technology continues to mature, methodical assessment across pollutant classes will remain essential for guiding substrate selection and advancing the application of this powerful technique in environmental protection and public health safety.
Evaluating Reusability, Stability, and Cost-Effectiveness of SERS Substrates
Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique for detecting environmental pollutants at ultratrace levels. The core of this technology lies in its substrates—nanostructured surfaces that amplify weak Raman signals by several orders of magnitude. For environmental monitoring applications, three critical factors determine the practical viability of SERS substrates: reusability, long-term stability, and overall cost-effectiveness. While traditional noble metal substrates offer excellent enhancement factors, they often suffer from limitations in these practical areas. Recent research has focused on developing alternative materials and innovative regeneration techniques to overcome these barriers. This guide provides a detailed comparison of substrate performance based on experimental data, offering environmental researchers a framework for selecting appropriate materials for specific application scenarios.
Comparative Performance Analysis of SERS Substrates
The evaluation of SERS substrates requires a multifaceted approach that considers enhancement capability alongside practical performance metrics. The following comparison synthesizes experimental data from recent studies to provide a comprehensive overview of available options.
Table 1: Comprehensive Comparison of SERS Substrate Performance Characteristics
| Substrate Type | Enhancement Factor (EF) | Reusability (Cycles) | Storage Stability | Key Advantages | Reported Limitations |
|---|---|---|---|---|---|
| Noble Metal (Au/Ag) | 10$^5$ - 10$^8$ [1] [89] | Limited (often single-use) | Moderate (oxidation issues) [89] | High, well-understood enhancement; commercial availability | High cost; susceptible to degradation; limited reusability |
| Transition Metal Nitrides (NbTiN) | 3.2 × 10$^4$ [90] | >5 cycles with two target molecules [90] | >6 months [90] | Exceptional thermal/chemical stability; cost-effective | Lower EF compared to optimized noble metals |
| 3D Nanostructures | >10$^8$ [1] | Varies by architecture | Good structural stability | High hot spot density; improved analyte capture | Complex fabrication; potential reproducibility issues |
| Ag/TiO2 Hybrid (PI-PC SERS) | Significantly enhanced vs. normal SERS [91] | Full renewal via photocatalysis [91] | Maintained after regeneration | Dual functionality: ultrasensitivity + self-cleaning | Requires UV irradiation protocol |
Table 2: Quantitative Detection Performance for Environmental Pollutants
| Substrate | Target Analyte | Limit of Detection (LOD) | Experimental Conditions | Reference |
|---|---|---|---|---|
| Ag/TiO2 (PI-PC SERS) | Methylene Blue | 1.02 × 10$^{-14}$ M [91] | UV pre-irradiation (PIERS) + post-cleaning | [91] |
| Ag/TiO2 (PI-PC SERS) | Thiram (pesticide) | 1.02 × 10$^{-11}$ M [91] | UV pre-irradiation (PIERS) + post-cleaning | [91] |
| Ag/TiO2 (Normal SERS) | Methylene Blue | 3.04 × 10$^{-11}$ M [91] | Without UV pre-irradiation | [91] |
| Ag/TiO2 (Normal SERS) | Thiram | 2.19 × 10$^{-9}$ M [91] | Without UV pre-irradiation | [91] |
| NbTiN Films | Rhodamine 6G | 10$^{-7}$ M [90] | One-step fabrication on YAO substrate | [90] |
Detailed Experimental Protocols and Methodologies
PI-PC SERS Protocol for Ultrasensitive and Renewable Detection
The Photo-Induced-Photo-Catalytic SERS (PI-PC SERS) technique represents a significant advancement in creating reusable substrates with exceptional sensitivity. The method synergistically combines photo-induced enhanced Raman scattering (PIERS) with photocatalytic self-cleaning on a single Ag/TiO2 nanocomposite platform [91].
Materials and Fabrication:
- Substrate Composition: Silver nanoparticles deposited on titanium dioxide (semiconductor) nanostructures [91]
- Preparation Method: Nanocomposite synthesis ensuring intimate metal-semiconductor interface
- Key Mechanism: Metal-semiconductor structure enabling both PIERS effect and photocatalytic activity
Experimental Workflow:
- Pre-irradiation Step: Substrate exposed to UV light (365 nm) before SERS measurements
- Analyte Exposure: Target molecules applied to activated substrate
- SERS Measurement: Raman spectra collection using standard instrumentation
- Post-irradiation Cleaning: UV exposure (365 nm, 60-70 minutes) to decompose analytes [91]
Enhancement Mechanism: UV pre-irradiation creates oxygen vacancies on the TiO2 surface, generating temporary defect energy levels at the metal-semiconductor interface. This significantly enhances charge transfer, boosting Raman scattering intensity compared to non-irradiated substrates [91].
Regeneration Efficiency: Photocatalytic decomposition completely removes analyte residues (confirmed by disappearance of characteristic Raman peaks), renewing the substrate for subsequent use without performance degradation [91].
Transition Metal Nitride Substrates for Long-Term Stability
Epitaxial Nb0.5Ti0.5N (NbTiN) films present an alternative approach to durable, reusable SERS substrates without photocatalytic functionality [90].
Fabrication Protocol:
- Deposition Method: Reactive radio frequency (RF) magnetron sputtering [90]
- Substrate Material: YAlO3 (YAO) single-crystalline substrate [90]
- Structural Characteristic: Nanoisland-like surface morphology via one-step fabrication
- Key Advantage: Simplified preparation avoiding complex multi-step processes
Stability Assessment Methodology:
- Reusability Testing: Multiple detection cycles (≥5) with different target molecules [90]
- Storage Stability: Performance evaluation over extended periods (≥6 months) [90]
- Regeneration Method: Conventional cleaning approaches between measurements
Performance Validation: Finite-difference time-domain (FDTD) simulations confirm electromagnetic field enhancement originates from nanoisland surface morphology, providing consistent SERS activity across reuse cycles [90].
Visualization of Workflows and Mechanisms
PI-PC SERS Mechanism and Workflow
PI-PC SERS Operational Cycle: This diagram illustrates the renewable detection process combining PIERS enhancement with photocatalytic cleaning.
Decision Framework for Substrate Selection
Substrate Selection Framework: A decision pathway for selecting optimal SERS substrates based on primary application requirements.
Essential Research Reagent Solutions
Successful implementation of SERS-based environmental detection requires specific materials and reagents tailored to the selected substrate technology.
Table 3: Essential Research Reagents for SERS Substrate Development and Application
| Reagent/Material | Function/Application | Specific Examples | Considerations for Environmental Detection |
|---|---|---|---|
| Plasmonic Metals | Electromagnetic enhancement | Silver nanoparticles, Gold nanostars [89] [52] | Silver offers higher enhancement; gold provides better stability [89] |
| Semiconductor Components | Charge transfer enhancement; photocatalytic activity | TiO2, ZnO [91] | Enables PIERS effect and self-cleaning capabilities [91] |
| Transition Metal Nitrides | Alternative plasmonic materials | NbTiN, TiN [90] | Superior chemical/thermal stability for harsh environments [90] |
| Functionalization Agents | Surface modification for selectivity | Thiols, silanes, aptamers [92] [52] | Improves target specificity in complex environmental matrices |
| Internal Standards | Signal normalization for quantification | Deuterated compounds, isotopically labeled analogs [92] | Essential for quantitative analysis across reuse cycles |
The ideal SERS substrate for environmental pollutant detection balances enhancement capability with practical considerations of reusability, stability, and cost. For applications demanding the highest sensitivity and where UV irradiation infrastructure is available, Ag/TiO2 PI-PC SERS substrates offer unparalleled performance with renewable functionality. For projects requiring exceptional longevity and chemical stability without complex regeneration protocols, transition metal nitrides like NbTiN offer compelling advantages. Traditional noble metal substrates remain relevant for applications where maximum enhancement is prioritized over reuse capability, while emerging 3D nanostructures show promise for capturing and concentrating dilute environmental contaminants. The selection framework presented herein enables researchers to match substrate technology to specific environmental monitoring scenarios, accelerating the implementation of SERS in practical pollution detection applications.
The Role of Artificial Intelligence and Machine Learning in Spectral Analysis and Validation
Surface-Enhanced Raman Spectroscopy (SERS) has emerged as a powerful analytical technique for detecting environmental pollutants, offering molecular fingerprinting capabilities with single-molecule sensitivity. The integration of Artificial Intelligence (AI) and Machine Learning (ML) is addressing long-standing challenges in SERS analysis, including spectral interpretation, quantification amidst complex matrices, and validation of results. This guide objectively compares the performance of various AI-ML approaches used with different SERS substrates for environmental sensing, providing researchers with experimental data and protocols to inform their substrate and algorithm selection.
SERS Enhancement Mechanisms and Substrate Design
SERS amplification originates from two primary mechanisms. The Electromagnetic Mechanism (EM) generates intense localized electromagnetic fields, or "hot spots," via localized surface plasmon resonance (LSPR) in plasmonic nanostructures, typically enhancing signals by factors of 10^4–10^8 [2] [1]. The Chemical Mechanism (CM) involves charge transfer between the analyte molecules and the substrate surface, contributing a smaller but specific enhancement [2].
Substrate architecture critically determines performance. Traditional 2D substrates (e.g., planar metal films) confine hot spots to a surface layer, while 3D substrates (e.g., vertically aligned nanowires, porous frameworks, and dendritic structures) distribute hot spots volumetrically, offering increased surface area and superior analyte accessibility [1]. Table 1 compares their characteristics.
Table 1: Comparison of 2D vs. 3D SERS Substrates
| Feature | 2D SERS Substrates | 3D SERS Substrates |
|---|---|---|
| Hot Spot Dimension | Confined to planar surface | Distributed volumetrically in all dimensions |
| Typical Enhancement Factor (EF) | 10âµâ€“10â· | >10⸠|
| Reproducibility | Moderate | High (RSD typically < 10%) |
| Analyte Accessibility | Limited diffusion on surface | Enhanced diffusion via 3D porous networks |
| Fabrication Methods | Lithography, self-assembly | Template growth, dealloying, freeze-drying |
Machine Learning Approaches for SERS Analysis
AI models for SERS are categorized into discriminative (classifying data, identifying patterns) and the emerging generative (creating new data, designing materials) models [93]. The workflow involves preprocessing, feature extraction, model training, and validation.
Common ML Algorithms and Applications
- Principal Component Analysis (PCA): An unsupervised algorithm for dimensionality reduction and data exploration. It helps visualize trends and remove noise from complex spectral datasets [93]. A model combining PCA with Optimal Class Discrimination and Compactness Optimization (OCDCO) achieved 97% accuracy for classifying serum spectra [94].
- Support Vector Machine (SVM) & Random Forest (RF): Supervised learning models for classification. RF, an ensemble method, often demonstrates superior performance. In detecting xylazine in illicit opioids, RF outperformed SVM, achieving 96% sensitivity and 88% specificity in a high-level data fusion approach [95].
- Explainable AI (XAI) and SHAP: Techniques like Shapley Additive Explanations (SHAP) bridge the "black box" gap in complex models (e.g., Extra Trees) by identifying the specific Raman bands responsible for classification, increasing confidence in the model's chemical validity [93].
- Convolutional Neural Networks (CNNs): Deep learning models that automatically learn hierarchical features from raw spectral data, showing great promise for complex pattern recognition [96].
Comparative Performance Analysis of AI-ML Models
The performance of an ML model is contingent on the specific SERS application, sample complexity, and the type of substrate used. The following experimental data highlights this interplay. Table 2 summarizes quantitative performance metrics of different AI/ML models applied to SERS analysis across various use cases.
Table 2: Performance Comparison of AI-ML Models in SERS Analysis
| Application | SERS Substrate | ML Model | Key Performance Metrics | Reference |
|---|---|---|---|---|
| Colorectal Precancer Detection | Microarray chip with Au/SnOâ‚‚ nanorope arrays | PCA-Optimal Class Discrimination and Compactness Optimization (OCDCO) | Accuracy: 97%Sensitivity: 95%Specificity: 97%AUC: 0.96 | [94] |
| Xylazine in Illicit Opioids | Not Specified | Random Forest (High-Level Fusion with IR data) | Sensitivity: 96%Specificity: 88%F1 Score: 92% | [95] |
| Support Vector Machine | Lower performance than Random Forest | [95] | ||
| Pathogen & Biomarker Detection | Various (e.g., lab-on-a-chip) | PCA + Linear Discriminant Analysis (LDA) | Effective for distinguishing six mycobacteria species | [93] |
| Exosome Classification | Various | Extra Trees with SHAP (XAI) | High accuracy; provides interpretable band assignment | [93] |
Experimental Protocols for SERS-Based Detection
Protocol 1: Detection of Organic Dyes using a Flexible PVDF Substrate
This protocol details the detection of Malachite Green (MG) and Rhodamine 6G (R6G), common organic pollutants, using a flexible and cost-effective substrate [97].
- Substrate Fabrication: A Polyvinylidene fluoride (PVDF) membrane is grafted with Multi-Walled Carbon Nanotubes (MWCNTs) using vacuum filtration. Subsequently, Au-Ag alloy nanoparticles (NPs) are incorporated onto the MWCNT/PVDF membrane via a dip-coating method. This creates a flexible substrate with abundant hotspots and synergistic EM/CM enhancement [97].
- SERS Measurement: The analyte solution (e.g., MG or R6G) is dispensed onto the Au-Ag/MWCNT/PVDF substrate. Measurements are conducted using a Raman spectrometer with a 785 nm laser, 5 mW power, and 10-second acquisition time. The flexible substrate allows for conformal contact with irregular surfaces, enabling practical sampling [97].
- AI/ML Analysis: The complex spectra, especially at low analyte concentrations, are processed using ML algorithms. The workflow typically includes background subtraction, normalization, and then application of models like PCA for feature reduction or RF for final classification and concentration prediction [97] [96].
Protocol 2: Serum Analysis for Medical Diagnostics using a Microarray Chip
This protocol, applicable to biomarker detection for diseases like cancer, emphasizes high-throughput and reproducible analysis [94].
- Substrate Fabrication: A microarray chip is fabricated using soft lithography with polydimethylsiloxane (PDMS) on a glass slide. The chip integrates a SERS-active substrate of Au/SnOâ‚‚ Nanorope Arrays (Au/SnOâ‚‚ NRAs), which is synthesized beforehand through a template-assisted method and Au deposition. The enclosed chip design protects the serum sample from degradation [94].
- SERS Measurement: A small volume (2 μL) of serum sample is dispensed into each microwell of the chip. SERS spectra are collected using a Raman microscope (e.g., 785 nm laser, 5 mW power, 50x objective) across a range of 600–1800 cmâ»Â¹. For each sample, spectra are acquired from multiple locations to ensure representativeness [94].
- AI/ML Analysis: The high-dimensional spectral data from healthy, precancerous, and cancerous serum samples is analyzed using the PCA-OCDCO model. PCA reduces the data dimensionality, and the OCDCO algorithm maximizes separation between different classes while optimizing the compact clustering of data points within the same class, leading to highly accurate diagnostic predictions [94].
The following diagram illustrates the core workflow of a SERS-AI analysis system.
SERS-AI Analysis Workflow
The Scientist's Toolkit: Essential Research Reagents and Materials
Successful SERS-based detection relies on a suite of specialized materials and reagents. Table 3 lists key components for developing and utilizing SERS substrates.
Table 3: Essential Research Reagents and Materials for SERS Sensing
| Material/Reagent | Function in SERS Experiment | Example Context |
|---|---|---|
| Gold (Au) & Silver (Ag) Salts | Precursors for synthesizing plasmonic nanoparticles that provide electromagnetic enhancement. | HAuCl₄·3H₂O for Au NPs in Au-Ag/MWCNT/PVDF substrate [97]. |
| Semiconductor Metal Oxides (e.g., SnOâ‚‚) | Form hybrid substrates; can provide chemical enhancement and stabilize metal NPs. | SnOâ‚‚ nanobowl arrays as a template for Au deposition [94]. |
| Polyvinylidene Fluoride (PVDF) | A flexible polymer membrane used as a support for creating bendable, permeable SERS substrates. | Base membrane for Au-Ag/MWCNT/PVDF flexible sensor [97]. |
| Multi-Walled Carbon Nanotubes (MWCNTs) | Enhance charge transfer (chemical mechanism), provide large surface area for analyte adsorption. | Grafted onto PVDF to improve SERS performance synergistically [97]. |
| Polydimethylsiloxane (PDMS) | A silicone-based polymer used for fabricing microfluidic chips and enclosures for SERS substrates. | Used to create the high-throughput microarray serum chip [94]. |
| Raman Reporter Molecules (e.g., R6G) | Used as standard probes for quantifying SERS substrate enhancement factor and performance. | Model analyte for testing the Au-Ag/MWCNT/PVDF substrate [97]. |
Performance Validation and Data Fusion Strategies
Robust validation is critical. Common practices include k-fold cross-validation (e.g., 5-fold) to optimize model parameters and prevent overfitting [95]. Data fusion with complementary techniques like Infrared (IR) spectroscopy is a powerful strategy. One study on xylazine detection combined SERS and IR data, with high-level fusion of Random Forest predictions (giving SERS a 90% voting weight) achieving an F1 score of 92%, outperforming single-technique models [95].
The diagram below outlines the process of comparing and selecting the optimal ML model, which is a critical step in validation.
ML Model Comparison and Validation
The synergy between advanced SERS substrates and tailored AI/ML models is pushing the boundaries of environmental pollutant detection. While 3D and flexible substrates provide superior sensitivity and practicality, the choice of ML algorithm—from robust options like Random Forest for fused data to interpretable models like PCA-OCDCO or XAI—depends heavily on the specific application and required balance between accuracy, interpretability, and scalability. Future directions will involve generative AI for substrate design and increased standardization, paving the way for deployable, intelligent SERS sensors for environmental monitoring.
Conclusion
The evaluation of SERS substrates reveals a technology at a pivotal point of transition from laboratory research to practical environmental application. The synergistic combination of novel nanomaterials, precise nanofabrication, and intelligent data analysis is paving the way for substrates that are not only extraordinarily sensitive but also reproducible, reliable, and deployable in the field. Key takeaways include the superior performance of hybrid materials that leverage both electromagnetic and chemical enhancement, the critical importance of 'hot spot' engineering for single-molecule detection, and the growing role of AI in overcoming spectral complexity. Future directions should focus on establishing standardized fabrication and validation protocols, developing inexpensive and portable devices for widespread monitoring, and exploring the integration of SERS with catalytic degradation for simultaneous detection and remediation of pollutants. For biomedical and clinical research, these advancements promise new tools for monitoring environmental exposures that impact human health, ultimately contributing to predictive toxicology and personalized medicine.
References
- 1. Three-Dimensional SERS Substrates: Architectures, Hot ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12467525/]
- 2. Research Progress of Surface-Enhanced Raman ... [https://www.mdpi.com/2304-6732/12/8/809]
- 3. SERS nose arrays based on a signal differentiation ... [https://www.nature.com/articles/s42004-025-01656-2]
- 4. Emerging trends in SERS-based veterinary drug detection [https://www.nature.com/articles/s41538-025-00393-z]
- 5. A practical approach to quantitative analytical surface ... [https://pubs.rsc.org/en/content/articlehtml/2025/cs/d4cs00861h]
- 6. SERS strategy for quantifying transparent dry analytes at ... [https://www.sciencedirect.com/science/article/pii/S0026265X25015462]
- 7. A Review on Surface-Enhanced Raman Scattering - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC6627380/]
- 8. Advances in Surface-Enhanced Raman Scattering Sensors of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10489740/]
- 9. Recent advances in the design of SERS substrates and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12009482/]
- 10. Why SERS Substrates Are Critical in Bio-Sensor ... [https://eureka.patsnap.com/report-why-sers-substrates-are-critical-in-bio-sensor-developments]
- 11. Improving Biomarker Detection with Surface-Enhanced ... [https://www.spectroscopyonline.com/view/improving-biomarker-detection-with-surface-enhanced-resonance-raman-scattering-an-interview-with-2025-charles-mann-award-winner-marc-porter-part-1-]
- 12. Experimental and Theoretical Comparison and Analysis of ... [https://www.mdpi.com/2076-3417/14/19/9040]
- 13. High-performance SERS substrate based on gold ... [https://www.sciencedirect.com/science/article/abs/pii/S2588842024000750]
- 14. Au-Ag Bimetallic Nanoparticles for Surface-Enhanced ... [https://www.mdpi.com/2304-8158/14/12/2109]
- 15. A review on nanomaterial-based SERS substrates for ... [https://www.sciencedirect.com/science/article/pii/S0048969724044000]
- 16. Ni/Ag bimetallic nanoparticle-engineered Ti3C2Tx MXene ... [https://pubmed.ncbi.nlm.nih.gov/41251548/]
- 17. Detection of pesticide residues using flower-like silver ... [https://pubmed.ncbi.nlm.nih.gov/39612096/]
- 18. Silver nanoparticles self assembly as SERS substrates with ... [https://pubs.rsc.org/en/content/articlelanding/2009/cp/b904744a]
- 19. A Novel Method for Rapid and High-Performance SERS ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11547355/]
- 20. Machine Learning-Assisted Sampling of SERS Substrates ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC8880398/]
- 21. The emerging role of semiconductor nano-photocatalysts in ... [https://pubs.rsc.org/en/content/articlehtml/2025/na/d5na00597c]
- 22. SERS Performance of Ti3C2Tx MXene-Based Substrates ... [https://www.mdpi.com/1996-1944/17/6/1385]
- 23. Au nanoparticle-engineered Ti3C2Tx MXenes as a high- ... [https://pubmed.ncbi.nlm.nih.gov/40047987/]
- 24. Surface-enhanced Raman spectroscopy as a tool for food ... [https://pubs.rsc.org/en/content/articlelanding/2025/na/d5na00539f]
- 25. Dual-functional magnetic MXene@Fe 3 O 4 @ag NPs with ... [https://www.sciencedirect.com/science/article/abs/pii/S0021979725019770]
- 26. Progress towards efficient MXene sensors [https://www.nature.com/articles/s43246-025-00907-y]
- 27. Recent advances in the functionalization of cellulose ... [https://www.frontiersin.org/journals/nanotechnology/articles/10.3389/fnano.2025.1599944/full]
- 28. Recent Advances in Surface-Enhanced Raman Scattering ... [https://www.mdpi.com/1424-8220/25/5/1370]
- 29. Lead-free halide double perovskite nanoflakes as high- performance ... [https://pubs.rsc.org/en/content/articlelanding/2025/nr/d5nr00437c]
- 30. Gradient SERS Substrates with Multiple Resonances for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9414786/]
- 31. Theoretical and experimental comparison of the ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10034477/]
- 32. A generalized exponential relationship between the ... [https://www.sciencedirect.com/science/article/pii/S0924424720307214]
- 33. Dynamic Imaging Analysis of SERS-Active Nanoparticle ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3660949/]
- 34. Development of affinity between target analytes and substrates in... [https://pubmed.ncbi.nlm.nih.gov/33226038/]
- 35. Recent Advances in SERS-Based Detection of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12610478/]
- 36. Fabricating a Three-Dimensional Surface - Enhanced ... Raman [https://www.mdpi.com/1424-8220/25/8/2575]
- 37. The emerging role of semiconductor nano-photocatalysts in... [https://pubs.rsc.org/en/content/articlelanding/2025/na/d5na00597c]
- 38. Detection of Organophosphorus, Pyrethroid, and ... [https://www.mdpi.com/2304-8158/14/14/2543]
- 39. Surface-enhanced Raman spectroscopy biosensor ... [https://link.springer.com/article/10.1007/s44462-025-00031-7]
- 40. A Review on Non-Noble Metal Substrates for Surface- ... [https://www.mdpi.com/2227-9040/11/8/427]
- 41. Precision Target Guide Strategy for Applying SERS into ... [https://www.intechopen.com/chapters/52021]
- 42. A rapid Surface-Enhanced Raman scattering method for ... [https://www.sciencedirect.com/science/article/abs/pii/S0026265X23013437]
- 43. Simultaneous Detection of Food Contaminants Using ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12427925/]
- 44. Highly sensitive and selective determination of uranyl ions ... [https://www.sciencedirect.com/science/article/abs/pii/S0039914024007860]
- 45. Review of SERS Substrates for Chemical Sensing - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC5485789/]
- 46. Innovative paper-based SERS substrate for rapid detection ... [https://www.sciencedirect.com/science/article/abs/pii/S1386142525001878]
- 47. Advances in Multifunctional Nanoagents and SERS-Based ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12026551/]
- 48. Research advances in SERS-based sensing platforms for ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12409673/]
- 49. Advancements and challenges on SERS-based ... [https://www.sciencedirect.com/science/article/abs/pii/S0924224424003480]
- 50. Silver coated porous silicon microarray SERS platform for ... [https://www.nature.com/articles/s41538-025-00532-6]
- 51. Simultaneous Detection of Food Contaminants Using ... [https://www.mdpi.com/2304-8158/14/17/2982]
- 52. Applications of surfaceâ€enhanced Raman spectroscopy in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10989676/]
- 53. Comparison of SERS Substrates to Conventional Detection ... [https://eureka.patsnap.com/report-comparison-of-sers-substrates-to-conventional-detection-methods]
- 54. Detection of Polystyrene Microplastics up to the Single ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12305665/]
- 55. Dual-substrate SERS sensor based on optical fiber for ultra ... [https://opg.optica.org/viewmedia.cfm?html=true&seq=0&uri=optcon-4-7-1505]
- 56. Advancing SERS-based detection of micro and ... [https://www.sciencedirect.com/science/article/pii/S2666154325007434]
- 57. Applications of Reproducible SERS Substrates for Trace ... [https://www.spectroscopyonline.com/view/applications-reproducible-sers-substrates-trace-level-detection]
- 58. Introduction and Development of Surface-Enhanced ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11510290/]
- 59. Improved Quantitative SERS Enabled by Surface Plasmon ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5981291/]
- 60. Electrochromic semiconductors as colorimetric SERS ... [https://www.nature.com/articles/s41467-019-08656-6]
- 61. Flexible surface-enhanced Raman scattering substrates [https://www.sciencedirect.com/science/article/abs/pii/S0010854525003091]
- 62. Trends in Application of SERS Substrates beyond Ag and ... [https://pubmed.ncbi.nlm.nih.gov/36354477/]
- 63. Quantitative SERS by hot spot normalization [https://pubs.rsc.org/en/content/articlelanding/2017/fd/c7fd00125h]
- 64. SERS mapping combined with chemometrics, for accurate ... [https://www.sciencedirect.com/science/article/pii/S1386142523012210]
- 65. - Surface spectroscopy as a tool for food and... enhanced Raman [https://pubs.rsc.org/en/content/articlehtml/2025/na/d5na00539f]
- 66. Online Integration of Paper Centrifugation with SERS for Sulfanilamide... [https://pubmed.ncbi.nlm.nih.gov/41116247/]
- 67. Enrichment strategies in surface - enhanced ... Raman scattering [https://www.oejournal.org/oes/article/cstr/32246.14.oes.2025.250015]
- 68. Ultralow-background SERS substrates for reliable ... [https://www.sciencedirect.com/science/article/abs/pii/S0304389423017910]
- 69. Surface-enhanced Raman scattering detection of ... [https://www.sciencedirect.com/science/article/abs/pii/S0026265X2402438X]
- 70. Comparison of surface-enhanced Raman scattering (SERS ... [https://www.sciencedirect.com/science/article/abs/pii/S003040261731032X]
- 71. Colloidal SERS | Dry Substrate | Reproducible Results [https://wasatchphotonics.com/applications/novel-sers-substrate/]
- 72. Advancements in SERS: Revolutionizing Biomedical Analysis ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12435236/]
- 73. Recent advances in the design of SERS substrates... [https://f1000research.com/articles/13-670]
- 74. Enhancement Factors of SERS Substrates Based on ... [https://link.springer.com/article/10.1007/s11468-025-03311-x]
- 75. Numerical Optimization Technique of Multilayer SERS ... [https://www.mdpi.com/2304-6732/11/1/12]
- 76. Gold vs. Silver Colloidal Nanoparticle Films for Optimized ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10216585/]
- 77. A study on a hybrid SERS substrates based on arrayed ... [https://www.sciencedirect.com/science/article/pii/S2238785422018907]
- 78. Study of SERS activity of different gold nanostructures ... [https://jmsg.springeropen.com/articles/10.1186/s40712-024-00191-7]
- 79. Surface-Enhanced Raman Scattering (SERS) Substrates ... [https://www.mdpi.com/2073-4360/15/12/2624]
- 80. Quantitative detection of trace nanoplastics (down to 50 nm ... [https://www.oejournal.org/article/doi/10.29026/oea.2025.240260]
- 81. Design and Experimental Demonstration of Pyramidal ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12163800/]
- 82. Decoding Mycotoxin Testing: Ensuring Food Safety with Advanced... [https://www.linkedin.com/pulse/decoding-mycotoxin-testing-ensuring-food-safety-methods-odhiambo-ucs1f]
- 83. A Mini-Review on Detection Methods of Microcystins - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC7601875/]
- 84. A comparison of ELISA and HPLC methods for ... [https://www.sciencedirect.com/science/article/abs/pii/S0278691513006480]
- 85. Screening, determination and confirmation of... | Semantic Scholar [https://www.semanticscholar.org/paper/Screening%2C-determination-and-confirmation-of-in-and-Shen-Jiang/d79e7441264e5873ac8a195a40222ad6217f32ed]
- 86. A comparison of ELISA and HPLC methods for ... [https://pubmed.ncbi.nlm.nih.gov/24036139/]
- 87. 1T/2H-MoS2 Nanoclusters as Plasmon-Free Surface- ... [https://pubmed.ncbi.nlm.nih.gov/40873344/]
- 88. SERS-powered precision: revolutionizing therapeutic drug ... [https://link.springer.com/article/10.1007/s00604-025-07670-4]
- 89. Surface Enhanced Raman Spectroscopy Substrate Market ... [https://www.24chemicalresearch.com/reports/139015/global-surfaceenhanced-raman-spectroscopy-substrate-market]
- 90. Reusability and long-life storage SERS substrate based on ... [https://www.sciencedirect.com/science/article/abs/pii/S0167577X25004434]
- 91. Synergizing PIERS and photocatalysis effects in a photo ... [https://pubs.rsc.org/en/content/articlehtml/2025/ra/d4ra07718k]
- 92. Biomedical SERS – the current state and future trends [https://pubs.rsc.org/en/content/articlehtml/2024/cs/d4cs00090k]
- 93. AI in SERS sensing moving from discriminative to generative [https://www.nature.com/articles/s44328-025-00033-2]
- 94. Machine learningâ€based SERS serum detection platform ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12478330/]
- 95. Optimized machine learning approaches to combine ... [https://pubs.rsc.org/en/content/articlelanding/2025/an/d4an01496k]
- 96. AI Boosts SERS for Next Generation Biomedical ... [https://www.spectroscopyonline.com/view/ai-boosts-sers-for-next-generation-biomedical-breakthroughs]
- 97. Flexible surface-enhanced raman scattering substrate ... [https://www.sciencedirect.com/science/article/abs/pii/S1876107025003347]